Highlights
- •
Residual tumor involvement in the trigone and urethra after radical cystectomy is associated with a higher tumor stage, and positive margins.
- •
It was significantly associated with tumor recurrence and cancer specific survival after RC, but not overall survival.
- •
Molecular characterization showed distinct CK4 expression in the bladder walls and the trigone/urethra in both humans and mouse models; negative CK4 expression in the bladder, focal positive CK4 expression in the human trigone and strong CK4 expression in the human prostatic urethra.
Abstract
Introduction
The trigone/urethra (T/U) has a distinct embryologic origin and a different histologic morphology compared to the rest of the urinary bladder. We sought to determine the association between tumors involved in the T/U and the presence of variant histology, pathologic, and oncologic outcomes in patients who underwent robot-assisted radical cystectomy (RARC).
Methods
Tumor location was classified into 2 groups: tumors in the bladder walls only, or tumors in the T/U area, with or without involvement of other bladder walls. Univariable and multivariable Cox regression models were used to determine the association between T/U with recurrence-specific ( RSS ), cancer-specific (CSS), and overall survival (OS).
Results
608 patients who underwent RARC were identified, T/U involvement occurred in 191 (31%). Patients in the T/U group were more likely to have pT3/pT4 (57% vs. 42%, P < 0.01), positive surgical margins (21% vs. 9%, P < 0.01), and received salvage chemotherapy more frequently (16% vs. 8%, P < 0.01). Squamous variant histology was more frequent in the T/U group (25% vs. 17%, P = 0.02). On multivariable analysis, T/U location was independently associated with RSS (HR1.63, 95% CI 1.23–2.16, P < 0.01) and CSS (HR1.50, 95% CI 1.04–2.16, P = 0.02) but not OS.
Conclusion
Residual T/U tumor involvement was associated with a higher risk of an advanced tumor stage, positive margin, cancer recurrence, and cancer-specific death.
1
Introduction
Radical cystectomy (RC) with pelvic lymph node dissection is the standard of care for patients with muscle-invasive bladder cancer (MIBC) and provides the best probability for long-term survival [ , ]. However, approximately 50% of patients develop disease recurrence after RC and eventually die from bladder cancer [ ]. Tumor stage and the presence of nodal metastasis are the most important factors for predicating local and distant recurrence, cancer specific and overall survival [ , ]. A few studies also suggest that intravesical tumor location at the time of RC may also help predict adverse pathology and bladder cancer survival. Previous series have shown that patients with tumors in the trigone have a higher risk of lymph node metastasis following radical cystectomy (RC) and have been associated with worse outcomes, suggesting that the tumor’s location might be an independent prognostic factor in the risk stratification for MIBC patients [ ]. However, the prognostic impact of tumor location on oncologic outcomes – especially with the lack of standardization of location classification – is lacking [ ].
The underlying biological reason for the difference between the tumors in the bladder walls and the tumors in the T/U remains unknown. Anatomically, the lower urinary tract urothelium is divided into different regions: the fundus (dome/wall), the bladder neck/trigone (a triangular-shaped region), and the urethra. The anatomic studies of the urinary bladder indicate significant differences within different regions, as shown in animal studies [ ]. The urinary bladder is embryologically derived from both endodermal and mesodermal tissues. The bladder walls originate from the upper end of the urogenital sinus, while the T/U is formed by the caudal ends of the mesonephric ducts. In addition, the upper urinary tract and the trigone bladder arise from the mesoderm during embryonic development. Patients with tumors in the trigone have up to a 6-fold higher risk for a synchronous tumor in the upper urinary tract [ ]. Therefore, to better understand the prognostic significance of intravesical tumor location, we compared the patients with tumors in the bladder only with the patients with tumors involved in the Trigone/Neck/Urethra as a group. Our objectives were to assess the impact of tumors in the T/U on adverse pathology, the presence of variant histology, recurrence, cancer specific survival, and overall survival following robot-assisted radical cystectomy (RARC).
2
Methods
A retrospective review of RARCs performed between 2005 and 2023 at a single institution was performed (I-79606). After excluding pT0 patients, 608 patients (pTis/pT1-2/pT3-4) were included in the final analyses. Tumor locations of all patients were documented in the final RC pathology reports by specialized genitourinary pathologists using standard methods, including the locations of dome, anterior wall, lateral wall, posterior wall, trigone, and urethra. All tumor locations were reported individually for multifocal tumors. Patients were classified into 2 groups according to the tumor location: tumors only in the bladder walls, or tumors involved in the (T/U) (with or without the bladder walls involved). Additional variant histology was defined by a histologic variant that was not found on the transurethral resection. Recurrence was defined as evidence of bladder cancer detected during follow-up, including distant and local recurrence. The first date of recurrence is considered when computing recurrence-specific survival . Both groups were then compared in terms of demographics, preoperative, operative, postoperative, pathologic, and oncologic outcomes.
Data were described in terms of median with interquartile range (IQR), frequency with percentage, Fischer’s exact test, or Wilcoxon rank-sum test. The median follow-up time was 4.6 years (IQR 2.5-8.1). A Kaplan–Meier curve was used to depict recurrence- specific ( RSS ), cancer-specific (CSS), and overall survival (OS) survival, and Log-rank test was used to assess significance. For RSS, time starts from cystectomy to the time of first recurrence or the last follow-up. For CSS, time starts from cystectomy until the patient’s death due to bladder cancer or last follow-up (patients censored if died due to other reason at last visit). For OS, time starts from cystectomy to death due to any cause or last follow-up. Multivariable COX regression models with forward selection were used to investigate variables associated with survival outcomes. Variables added to the multivariable model: Age, gender, body mass index (BMI), race, American Society of Anesthesiology (ASA) score, Charleston Comorbidity Index, previous radiation, previous abdominal surgery, preoperative hydronephrosis, approach and type of urinary diversion, Intensive Care Unit (ICU) admission, inpatient stay, lymph node yield, estimated blood loss, transfusion, operative time, histology including variant histology, tumor grade, pT, pN+, positive surgical margins, and Trigone/Urethra involvement. All analyses were double-sided with a significance level of 0.05. Analysis was performed with SAS 9.4.
3
Results
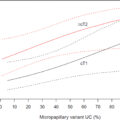
The final cohort consisted of 608 patients (191 with T/U location, 417 with bladder wall) after excluding pT0 patients. The median age was 70 years (IQR 62-76). Median follow-up time was 4.6 years (IQR 2.5-8.1). Preoperative hydronephrosis occurred in 116 (19%). Patients in the T/U group were more likely to have preoperative hydronephrosis (28% vs. 15%, P < 0.001), positive surgical margin (21% vs. 9%, P < 0.001), P ≥T3 stage (57% vs. 42%, P = 0.001). Squamous variant histology was more frequent in the T/U group (25% vs. 17%, P = 0.02), but there was no significant difference in the presence of any variant histology (55% vs. 49%, P = 0.19), or pN+ (33% vs. 26%, P = 0.07) ( Table 1 ).
| Variable | Walls | Trigone/Urethra | All | P Value | |
|---|---|---|---|---|---|
| Number of Patients, n (%) | 417 (69) | 191 (31) | 608 | – | |
| Age, years, median (IQR) | 70 (62–76) | 70 (60–76) | 70 (62–76) | 0.68 | |
| Male, n, (%) | 307 (74) | 139 (73) | 446 (73) | 0.84 | |
| BMI, Kg/m 2 , median (IQR) | 28 (25–32) | 28 (24–33) | 28 (25–32) | 0.71 | |
| Black race, n (%) | 14 (3) | 8 (4) | 22 (4) | 0.64 | |
| ASA score ≥3, n (%) | 247 (61) | 106 (58) | 353 (60) | 0.59 | |
| Charleston comorbidity index, median (IQR) | 5 (4–6) | 5 (4–6) | 5 (4–6) | 0.39 | |
| Previous radiation, n (%) | 20 (5) | 15 (8) | 35 (6) | 0.14 | |
| Previous abdominal surgery, n (%) | 223 (54) | 108 (57) | 331 (55) | 0.54 | |
| cT3/T4, n (%) | 37 (9) | 26 (14) | 63 (11) | 0.08 | |
| Preoperative Hydronephrosis, n (%) | 63 (15) | 53 (28) | 116 (19) | < 0.0001 | |
| Intracorporeal urinary diversion, n (%) | 327 (80) | 147 (78) | 474 (79) | 0.67 | |
| Neobladder, n (%) | 35 (8) | 12 (6) | 47 (8) | 0.42 | |
| ICU admission, n (%) | 267 (64) | 127 (66) | 394 (65) | 0.58 | |
| Inpatient stay, days, median (IQR) | 7 (6–10) | 7 (6–10) | 7 (6–10) | 0.96 | |
| LN yield, median (IQR) | 22 (15–28) | 22 (15–28) | 22 (15–28) | 0.89 | |
| Estimated blood loss ml, median (IQR) | 200 (100–400) | 200 (100–500) | 200 (100–400) | 0.89 | |
| Transfusion, n (%) | 17 (4) | 12 (6) | 29 (5) | 0.23 | |
| Operating time hours, median (IQR), hours | 5.85 (4.85–7.02) | 6.08 (4.97–7.13) | 5.9 (4.09–7.07) | 0.21 | |
| Pure urothelial histology, n (%) | 214 (51) | 85 (45) | 299 (49) | 0.41 | |
| Single variant histology, n (%) | 147 (35) | 74 (40) | 221 (37) | ||
| More than one variant histology, n (%) | 56 (14) | 28 (15) | 84 (14) | ||
| Pre/Post Variant Histology Involvement Sub-types, n (%) | Squamous Cell | 70 (17) | 48 (25) | 118 (19) | 0.02 |
| Micropapillary | 38 (9) | 19 (10) | 57 (9) | 0.77 | |
| Adenocarcinoma | 40 (10) | 16 (8) | 56 (9) | 0.76 | |
| Sarcomatoid | 37 (9) | 14 (7) | 51 (8) | 0.64 | |
| Nested | 17 (4) | 7 (4) | 24 (4) | 1.00 | |
| Plasmacytoid | 15 (4) | 7 (4) | 22 (4) | 1.00 | |
| Small Cell | 9 (2) | 4 (2) | 13 (2) | 1.00 | |
| Neuroendocrine | 7 (2) | 5 (3) | 12 (2) | 0.53 | |
| Giant Cell | 7 (2) | 4 (2) | 11 (2) | 0.75 | |
| Signet Cell | 7 (2) | 4 (2) | 11 (2) | 0.75 | |
| Lymphoepithelial | 6 (1) | 1 (1) | 7 (1) | 0.44 | |
| Clear Cell | 5 (1) | 1 (1) | 6 (1) | 0.67 | |
| Leiomyosarcoma | 1 (<1) | 1 (1) | 2 (<1) | 0.53 | |
| Microcystic | 2 (<1) | 0 (0) | 2 (<1) | 1.00 | |
| Rhabdoid | 1 (<1) | 1 (1) | 2 (<1) | 0.53 | |
| Columnar Differentiation | 1 (<1) | 0 (0) | 1 (<1) | 1.00 | |
| Trophoblastic Differentiation | 1 (<1) | 0 (0) | 1 (<1) | 1.00 | |
| Other | 7 (2) | 2 (1) | 9 (1) | 0.73 | |
| pT3/T4, n (%) | 175 (42) | 108 (57) | 283 (47) | 0.001 | |
| pN+, n (%) | 107 (26) | 63 (33) | 170 (28) | 0.07 | |
| Positive surgical margins , n (%) | 36 (9) | 40 (21) | 76 (13) | < 0.0001 | |
| Postoperative adjuvant chemotherapy, n (%) | 42 (10) | 25 (13) | 67 (11) | 0.33 | |
| Postoperative salvage chemotherapy, n (%) | 34 (8) | 30 (16) | 64 (11) | 0.007 | |
| Cystectomy era (Era 1 before 2010), n (%) | 102 (24) | 50 (26) | 152 (25) | 0.35 | |
| Cystectomy era (Era 2 2011-2014), n (%) | 94 (23) | 41 (21.5) | 135 (22) | ||
| Cystectomy era (Era 3 2015-2018), n (%) | 114 (27) | 41 (21.5) | 155 (26) | ||
| Cystectomy era (Era 4 2019), n (%) | 107 (26) | 59 (31) | 166 (27) | ||
| High grade complications, n (%) | 194 (47) | 105 (55) | 299 (49) | 0.06 | |
| Readmission, n (%) | 206 (49) | 104 (54) | 310 (51) | 0.26 | |
| Recurrence , n (%) | 118 (28) | 91 (48) | 209 (34) | < 0.0001 | |
| Bladder cancer death , n (%) | 68 (18) | 54 (32) | 122 (23) | 0.001 | |
| Death from any cause , n (%) | 175 (42) | 104 (54) | 279 (46) | 0.005 | |
| Follow up years, median (IQR) | 4.7 (2.5,8.1) | 3.9 (2.5,8.0) | 4.6 (2.5,8.1) | 0.65 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree