Highlights
- •
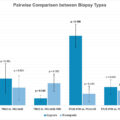
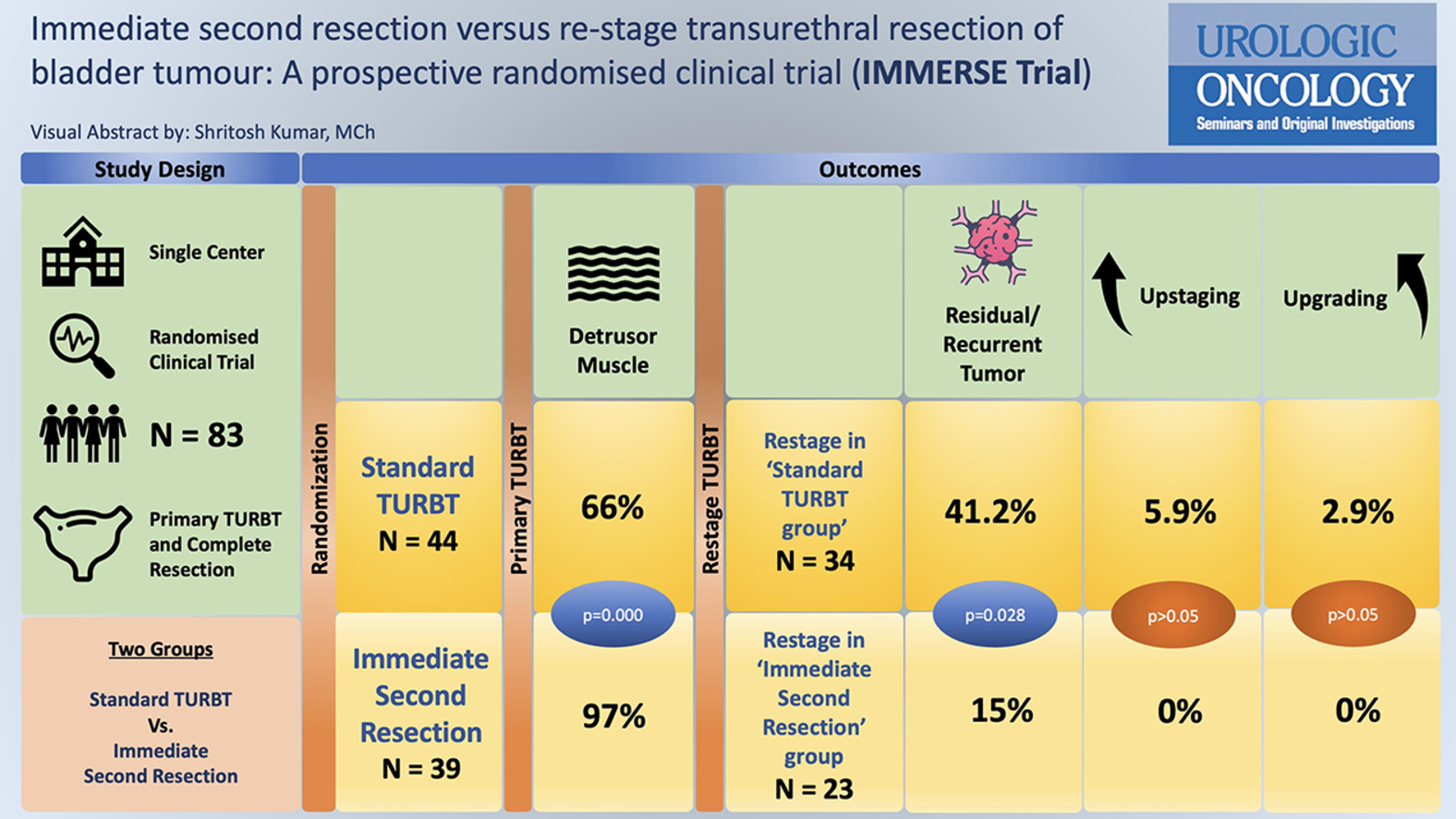
Detrusor muscle sampling rates are significantly higher in patients undergoing immediate second resection than standard TURBT (97% vs. 66%).
- •
There is a high rate of recurrence in both groups (Standard TURBT – 41% vs. immediate second resection – 17%), suggesting the role of second (restage) TURBT in patients with HG and/or T1 tumors.
- •
Presence of high rates of residual/ recurrent tumors at restage signify the role of improved detection and better surgical resection quality during primary TURBT.
Abstract
Background
The role of repeat transurethral resection of bladder tumor (TURBT) for the management of nonmuscle invasive bladder carcinoma is debated, especially when initial resections include detrusor muscle. This study compares immediate second resection (additional deep biopsies in the same session) with standard restage TURBT performed 2–6 weeks post-initial TURBT to determine adequacy in detrusor muscle sampling and compare the disease rate at restage TURBT in both groups.
Material and Methods
A randomized trial was conducted at a tertiary care hospital, including patients aged ≥18 years undergoing TURBT with complete primary tumor resection. Cases were randomized into two groups i.e., ‘standard TURBT’ (complete tumor resection with a deep biopsy) and “immediate second resection” (complete tumor resection, deep biopsy and additional deep biopsies). The primary endpoint was the presence of detrusor muscle in biopsy specimens, analyzed by a single pathologist. Secondary endpoints included perioperative complications, residual/ recurrent tumors, and factors affecting these recurrences.
Result
The study included 83 patients: 44 in the ‘standard TURBT’ group and 39 in the ‘immediate second resection’ group. The detrusor muscle was present in 66% of standard TURBT cases and 97% of immediate second resection cases, showing a statistically significant improvement ( P = 0.000). Residual disease was found in 41% of restage TURBT patients in the standard group and 15% in the immediate second resection group, the majority being high-grade and T1 tumors ( P = 0.028). There were no significant differences in tumor grade or perioperative complications between the groups. However, immediate second resection showed 18% higher detrusor muscle sampling rates than standard re-stage TURBT done at 2–6 weeks ( P = 0.021).
Conclusion
Immediate second resection at the time of initial TURBT significantly improves detrusor muscle sampling rates and decreases residual tumors at restage. Despite higher muscle sampling, a considerable proportion of patients still exhibited residual or recurrent tumors in both groups, emphasizing the need for improved detection and biopsy techniques during primary TURBT.
Graphical abstract
1
Introduction
Bladder cancer is the seventh most common malignancy among males worldwide and the tenth commonest when both genders are included [ ]. Disease involving the mucosa (stage Ta, CIS) or submucosa (stage T1) accounts for 75% of all bladder cancer cases, and this proportion is even higher in patients less than 40 years of age [ ]. Non-muscle invasive bladder carcinoma (NMIBC) denotes a group definition that includes Ta, CIS, and T1 tumors. The goal of transurethral resection of bladder tumor (TURBT) in clinical NMIBC is to completely remove all visible lesions and to obtain a correct histology and pathological staging. Detrusor muscle in the specimen is an important criterion that serves as an indicator of resection quality and deep biopsy is recommended in all bladder tumor resections to include the detrusor muscle (except in known or recurrent LG or TaG1 tumors [ ].
The role of repeat TURBT is well accepted for incomplete endoscopic resections, where detrusor muscle is not identified on histopathology. However, retrospective studies have questioned the role of re-stage TURBT (usually done within 2–6 weeks after initial TURBT) in the setting of complete resections, with no improvement in outcomes of patients with T1HG lesions if the muscle was present in the specimen of primary TUR [ ]. While previous studies have shown the risk of upstaging and the presence of residual or recurrent disease at restage, its role in the setting of adequate muscle and complete resection at initial TURBT has been questioned [ ].
Since a re-stage procedure entails another anesthesia and surgery session, performing a repeat procedure heralds associated complication risks as well. Furthermore, it increases the burden on the healthcare infrastructure and, also adds to an extraneous burden on the patient, both financially and mentally.
To address this gap of high-quality evidence in patients with NMIBC undergoing re-stage TURBT after 2-6 weeks and its role in adequate staging and further long-term outcomes, this study was designed in a prospective and randomized manner to compare immediate second deep biopsy (additional deep biopsies in the same anesthesia session after completing TURBT along with standard deep biopsies) with standard delayed re-stage TURBT after 2–6 weeks. The study hypothesized that taking an additional deep biopsy at the time of initial TURBT would be akin to doing a biopsy at 2–6 weeks from the tumor base, and would be equivalent in terms of detrusor muscle sampling rate.
2
Methods
2.1
Study design and participants
IMMERSE was a randomized, phase 2 trial conducted at a tertiary care hospital from January 2023 to December 2023. The trial was designed to compare the detrusor muscle sampling rates during primary TURBT, immediate second resection (at the time of primary TURBT), and at restage TURBT.
Patients undergoing TURBT who achieved complete resection of the primary bladder tumor and were aged ≥18 years were included. Consent for inclusion was taken before taking the patient for surgery. Exclusion criteria included non-regional lymph node or distant metastasis on cross-sectional imaging or chest X-ray, incomplete resection or deep biopsy not taken (in anticipation of radical cystectomy), clinical stage ≥cT3, single recurrent tumor less than 1cm in size with previous low-grade histology, perforation during TURBT or abandonment of procedure for any reason, patients with severe comorbidities (American Society of Anaesthesiologists Score >3), and pregnant or lactating patients.
2.2
Randomization
Randomization was done using a computer-generated randomization schedule and random permuted blocks (of lengths 4). Sequentially numbered, opaque, sealed envelopes were used for the allocation concealment. The trial was registered with the Clinical Trials Registry of India (CTRI/2023/01/048778) after due ethics committee approval.
2.3
Procedures
Patients with bladder tumors fulfilling the inclusion criteria and undergoing an initial TURBT with complete resection (including a separate deep biopsy) were randomized into 2 groups. Completion of TURBT was defined as complete resection of the visible tumor as per surgeon’s discretion and collection of separate deep biopsy chips. Allocation to study groups was done at this time (once the operating surgeon had satisfactorily completed the procedure) by the nursing staff who opened the envelope and informed the surgeon regarding the group allocation.
In the first group i.e., ‘Standard TURBT’, the procedure was stopped at this point only and 2 specimens were sent for histopathology (‘TURBT chips’ and ‘Deep Biopsy’). In the second group, patients underwent additional deep biopsies from the tumor bed in the same sitting from the base and margins. A minimum of 3 chips were taken as an adequate sample for the second resection and 3 biopsy specimens were sent (‘TURBT chips’, ‘Deep biopsy’, and ‘Additional Deep Biopsy’). The samples were evaluated histologically and patients with no detrusor muscle in the initial specimen (except Ta-LG tumors) or T1 and/or HG tumors underwent a ‘re-stage TURBT’ at 2-6 weeks intervals. All cases were done by residents or fellows under supervision using a bipolar resection system in saline ( Fig. 1 ).
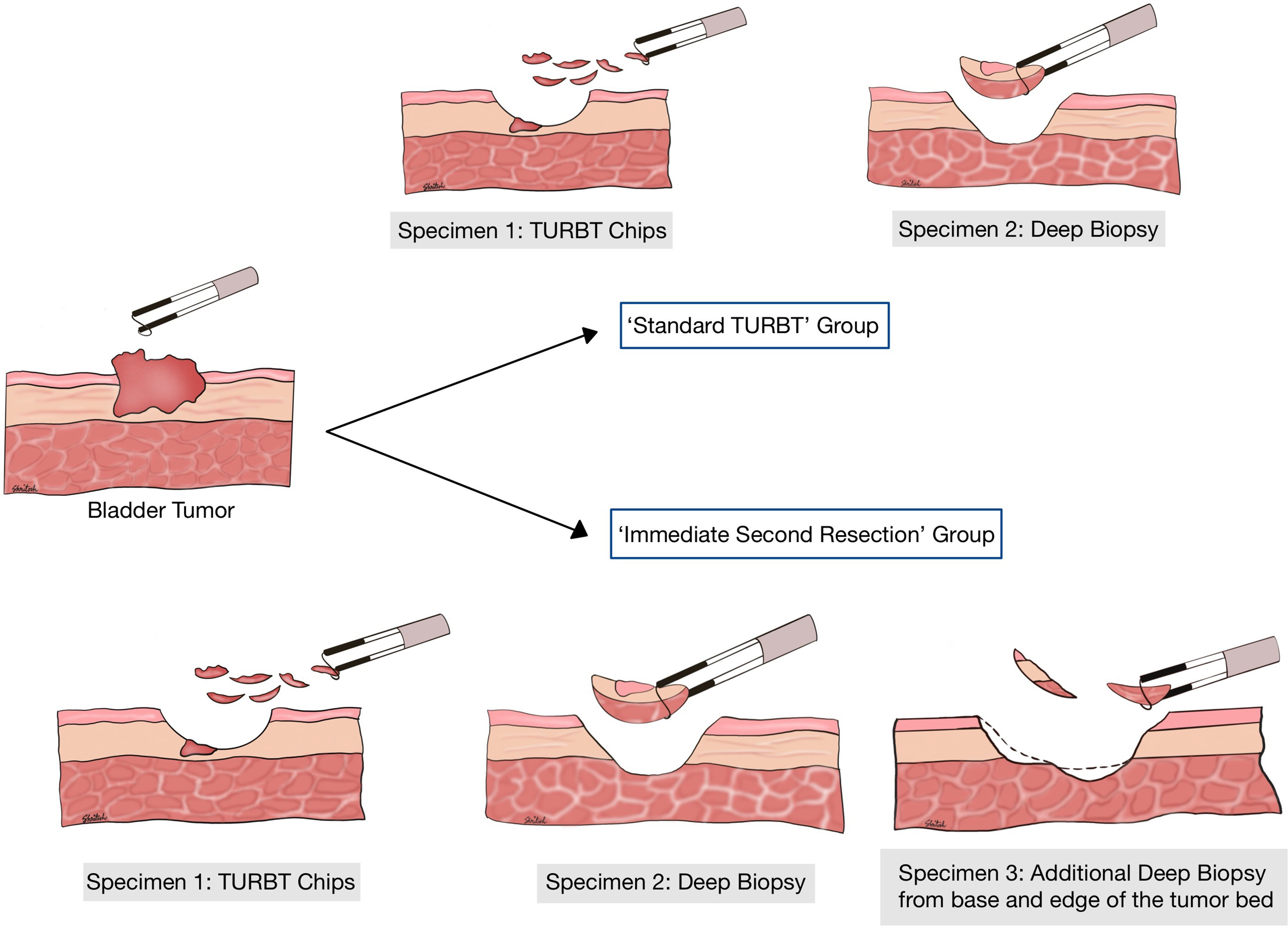
All the biopsy specimens were analyzed by a single pathologist. The presence of detrusor muscle in biopsy was noted as the primary end-point and compared between the 2 groups as per appropriate statistical methods. Any perforation or post-operative complications were recorded and compared between the 2 groups as secondary endpoints. All patients who were presumed to be non-muscle invasive by the surgeon at the end of the first surgery received a single dose of intravesical mitomycin C or Gemcitabine perioperatively. Patients in the low-risk category as per European Association of Urology (EAU) guidelines only received 1 dose of perioperative intravesical chemotherapy. Intermediate-risk patients in addition to the single peri‑operative dose received 1 year of adjuvant chemotherapy or intravesical bacillus Calmette-Guerin (BCG). High-risk patients were administered intravesical full-dose BCG for 1–3 years. Further surveillance was continued as per the EAU guidelines, the data of which were not included in this present study.
2.4
Sample size
The study was designed to test whether ‘Immediate second resection’ was as good as ‘Standard restage’ by a small pre-defined margin. In clinical terms, obtaining additional biopsies from the tumor base at the time of primary TURBT would hypothetically generate more detrusor muscle samples. For statistical power and significance, the margin for this trial was taken as 15%, meaning ‘Immediate second resection’ would at least give 15% more detrusor muscle than the ‘standard restage’ for it to be considered equivalent. A total of 72 evaluable patients would be needed (36 patients per arm) with 80% power and a significance level (α) of 5%. Assuming an attrition rate of 20%, the required sample size to conduct the trial was 86 (43 patients in each arm).
2.5
Outcomes
2.5.1
Primary objective
To compare the adequacy of staging/resection in terms of detrusor muscle (DM) sampling obtained during immediate second resection versus standard restage TURBT.
2.6
Secondary objectives
- 1.
To find disease (persistent disease/residual tumors and recurrences) rate at restage TURBT in both groups.
- 2.
To compare perioperative complications between the 2 groups.
- 3.
To evaluate factors associated with tumor recurrence.
2.7
Statistical analysis
Immediate second resection and restage groups were compared for the relative number of patients in each category. Data was processed using Statistical Package for Social Scientists (SPSS version 25.0 for Windows, SPSS Inc., Chicago, IL, United States). Continuous data were expressed as mean ± SD for normally distributed data or median (interquartile range) for skewed data. For skewed data, the Mann-Whitney U test was used, or else, the student’s t-test was used to compare the 2 groups. Categorical variables were analyzed using the chi-square test or Fisher’s exact test. A P -value of < 0.05 was considered statistically significant.
3
Results
A total of 83 patients were included in this study. The ‘Standard TURBT’ group (Group 1) had 44 patients while the ‘Immediate Second Resection’ group (Group 2) had 39 patients. The preoperative baseline patient characteristics were similar across both groups ( Table 1 ). Based on the histopathology of initial TURBT, restage was done in 77% and 59% of patients in Groups 1 and 2 respectively ( P = 0.437) ( Fig. 1 ).
| Variables | Standard TURBT ( n = 44) | Immediate Second resection ( n = 39) | P value |
|---|---|---|---|
| Age (Years) | 61.66 ± 13.82 | 58.67 ± 13.02 | 0.313 |
| Gender | 0.162 | ||
| 37 | 37 | |
| 7 | 2 | |
| Smokers | 22 | 15 | 0.291 |
| Comorbidities | |||
| 20 | 15 | 0.723 |
| 11 | 12 | |
| 5 | 7 | |
| 2 | 2 | |
| 5 | 2 | |
| 1 | 1 | |
| Tumor focality | |||
| 30 | 29 | 0.535 |
| 14 | 10 | |
| Maximal tumor diameter | |||
| 20 | 15 | 0.519 |
| 24 | 24 | |
| Need for Restage Procedure | 34 (77%) | 23 (59%) | 0.437 |
3.1
Primary TURBT
In the standard TURBT group, detrusor muscle was present in 66% of cases, whereas in the immediate second resection group, 82% of cases had detrusor on deep biopsy and 97% on additional deep biopsy ( Table 2 ), which was statistically significant at P = 0.000. There was no difference between the groups in terms of pathological T stage, WHO tumor grade, and complications.
| Variables | Standard TURBT ( n = 44) | Immediate Second resection ( n = 39) | P value | |
|---|---|---|---|---|
| Histopathology | ||||
| LG Ta | 6 | 10 | 0.264 a | |
| LG T1 | 12 | 7 | ||
| HG Ta | 0 | 1 | ||
| HG T1 | 21 | 16 | ||
| HG T2 | 2 | 4 | 0.412 c | |
| No malignancy | 2 | 0 | ||
| PUNLMP | 0 | 1 | ||
| Cystitis Cystica | 1 | 0 | ||
| Detrusor muscle | Deep biopsy | Additional deep biopsy | ||
| 15 | 7 | 1 | 0.096 b |
| 27 | 27 | 34 | |
| 2 | 5 | 4 | |
| % Muscle present | 66% | 82% | 97% | 0.000 d |
| Variant histology | ||||
| 0 | 1 | ||
| 0 | 1 | ||
| pT stage | ||||
| 6 (13.6%) | 11 (28.2%) | 0.148 | |
| 33 (75%) | 23 (58.9%) | ||
| 2 (4.5%) | 4 (10.2%) | ||
| 3 (6.8%) | 0% | ||
| 0% | 1 (2.5%) | ||
| Grade (WHO 2022) | ||||
| 18 (40.9%) | 17 (43.6%) | 0.940 | |
| 23 (52.2%) | 21 (47.6%) | ||
| Complications | ||||
| 2 | 1 | ||
| 0 | 0 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree