Highlights
- •
Increasing cumulative smoking exposure is associated with worse oncologic and overall survival outcomes.
- •
For moderate to heavy smokers, cessation did not appear to improve survival outcomes.
- •
Despite worse noncancer mortality, smokers were still at high risk of worse cancer survival compared to nonsmokers.
Abstract
Background
The impact of cumulative smoking exposure (CSE) on oncologic outcomes for upper tract urothelial carcinoma (UTUC) remains understudied. We examined the effect of this factor on oncologic outcomes in UTUC patients undergoing radical nephroureterectomy utilizing a large contemporary multicenter, multinational cohort.
Methods
Multicenter review of 1,730 patients across 17 institutions. A total of 1,041 patients met selection criteria: nephroureterectomy for urothelial carcinoma without variant histology and complete pathologic and smoking data. Smoking exposure was stratified as light, moderate, or heavy by cigarettes per day and years smoking based on prior studies. Cancer-specific (CSS) and overall survival (OS) were assessed using Kaplan–Meier and multivariable hazards models. A sub-analysis examined the effect of smoking cessation on survival stratified by CSE.
Results
Median follow-up (IQR) was 24 (10–48) months. Light CSE was equal to a median of 2.0 pack years smoked, moderate CSE was equivalent to 13.0 pack years, and heavy CSE was equivalent to 40 pack-years. Five-year CSS and OS were 97% and 91% in nonsmokers, 96% and 89% with light exposure, 85% and 66% with moderate exposure, and 75% and 60% with heavy exposure. On multivariable hazards models, both moderate and heavy smoking exposure were associated with worse CSS and OS compared to nonsmokers. Smoking cessation was not associated with improved survival outcomes among patients with moderate or heavy CSE.
Conclusions
Increasing CSE was associated with worse general health and oncologic outcomes in this UTUC cohort. Smoking cessation can modulate cancer outcomes up to certain thresholds of smoking exposure, emphasizing the need for both early smoking cessation and continued aggressive cancer treatment in patients with UTUC.
1
Introduction
While the link between smoking and development of upper tract urothelial carcinoma (UTUC) is clear, the oncologic impact of cumulative smoking exposure (CSE) is not well studied [ ]. A few multi-institutional and high-volume single-center studies have investigated the impact of CSE on UTUC outcomes with mixed results [ ]. Ehdaie et al. [ ] evaluated smoking on outcomes of UTUC undergoing radical nephroureterectomy (RNUx) and found current smokers had worse noncancer-related survival, but similar cancer recurrence rates compared to former or never smokers. This highlights the impact of other competing risks on noncancer mortality in this population. In contrast, Rink et al. [ ] investigated 864 patients who underwent RNUx for UTUC and found that current smoking status and heavy CSE were associated with advanced disease, disease recurrence, and cancer-specific survival (CSS). Importantly, the two largest studies on CSE and UTUC survival outcomes did not include patients receiving neoadjuvant chemotherapy (NAC) and only had small numbers of patients receiving adjuvant chemotherapy (AC), both of which are now critical components of contemporary management strategies [ ].
Given discrepant results in existing literature, there remains a need to better understand both the oncologic risks and competing risk of noncancer-related death in this population, particularly in the era of NAC and AC. Such information could inform prognostication and direct appropriate counseling on smoking cessation to improve oncologic outcomes. As such, we examined the impact of CSE on oncologic outcomes in patients with UTUC undergoing RNUx utilizing a large contemporary, multicenter, multinational cohort.
2
Methods
2.1
Data source
The ROBotic surgery for Upper tract Urothelial cancer STudy (ROBUUST 2.0) is a multicenter, retrospective dataset comprising 17 tertiary hospitals examining outcomes for UTUC from 2005 to 2022. After Institutional Review Board approval and a data sharing agreement, de-identified data were collected and shared among the investigators.
2.2
Patient selection, variables, and outcomes of interest
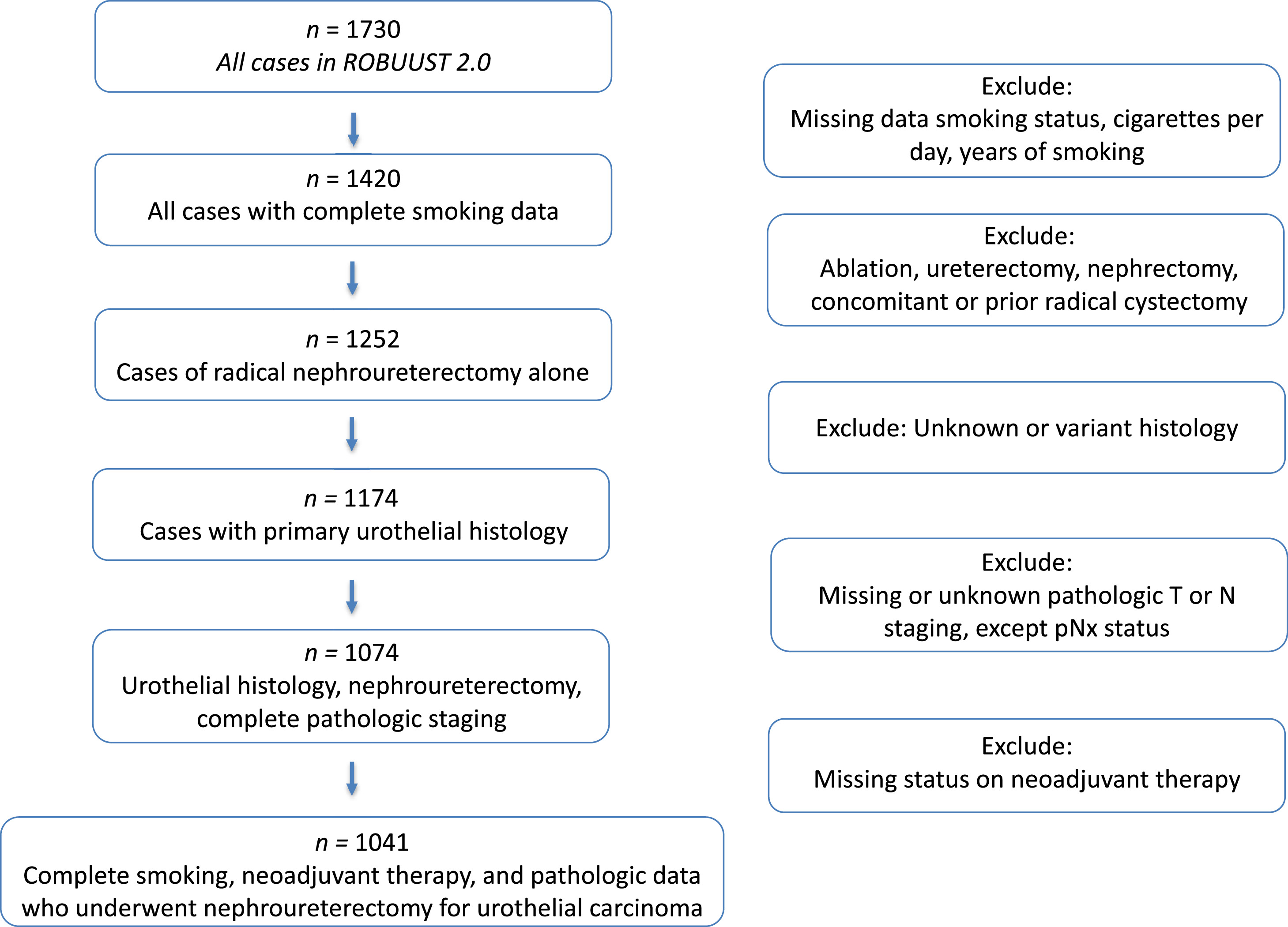
Fig. 1 shows our selection schema. Patients with missing data on smoking exposure, including current smoking status, cigarettes per day, and years of smoking ( n = 310) were excluded from analysis. Patients were stratified by CSE based on prior studies [ ]. CSE was defined as nonsmokers, light exposure (≤19 cigarettes per day and ≤19 years smoking), or heavy exposure (>20 cigarettes per day and >20 years smoking), with all other cases being moderate exposure [ ]. This definition of CSE was chosen a priori to allow for comparison between our current study and the previously reported largest study examining the effect of smoking on UTUC [ ]. Data regarding current smoking status at time of RNUx, cigarettes per day, and years of smoking was determined by clinical chart review at each individual center; there was no data regarding years since cessation for former smokers. We additionally excluded patients who did not undergo RNUx, nonurothelial histology, missing clinical tumor or nodal staging, and missing data on receipt of neoadjuvant therapy.
Demographic variables on patient age, gender, and comorbidities were collected. Cancer-specific variables included history of transurethral resection of bladder tumor, preoperative hydronephrosis, tumor multifocality, location, size, and perioperative intravesical therapy at RNUx. Pathologic variables included pathologic T and N stage, grade, lymphovascular invasion, and necrosis. Cisplatin-based NAC was defined as receipt of either a combination of gemcitabine and cisplatin vs. a combination of methotrexate, vinblastine, doxorubicin, and cisplatin. Noncisplatin-based regimens used carboplatin, immunotherapy, a combination of these, or was specific regimen was unavailable in some cases. Platinum-based AC was defined as receipt of any platinum-containing (cisplatin or carboplatin) regimen.
Our main outcomes of interest were CSS and overall survival (OS). Secondary survival outcomes included intravesical or extravesical recurrence free survival (RFS). Each of these outcomes were stratified by CSE.
2.3
Statistical analysis
Statistical analyses were performed using Stata, version 18.0 (College Station, TX USA). For demographic analysis, Chi-squared testing and Kruskal–Wallis testing was performed for categorical and continuous variables, respectively. The Kaplan–Meier (KM) method and competing risk regression was used for univariable analysis of survival outcomes (RFS, CSS, OS) and CSE. To examine whether smoking cessation modulated the effect of CSE on survival outcomes, an additional KM survival analysis was performed stratifying CSE by current vs. former smoking status in order to more carefully examine the combined impact of persistent smoking and increasing CSE on survival outcomes. Multivariable competing risk regression analysis examined the relationship between CSE and recurrence or CSS adjusting for the competing risk of noncancer death. OS was assessed using multivariable Cox hazards models. For the multivariable model, variance inflation factor scores were measured to assess the degree of collinearity between variables in the final survival model; all scores were between 1.0 and 1.64 suggesting minimal levels of collinearity ( Supplementary Table 2 ). All analysis were two-sided and considered statistically significant at P < 0.05.
3
Results
3.1
Patient characteristics
There were 1,041 patients meeting criteria for analysis ( Fig. 1 ). Nonsmokers made up 56% ( n = 586) of the cohort, followed by heavy CSE, moderate CSE, and light CSE ( Table 1 ). The number of current active and former smokers was similar between light, moderate, and heavy CSE (51.9% vs. 43.5% vs. 49%, P = 0.4 for subgroup). Median pack years smoked was 2.0 for light CSE, 10.0 for moderate CSE, and 40.0 for heavy CSE ( Table 1 , P < 0.001). Patients with heavy or light CSE were younger. Of the overall cohort, 95% of all patients would be stratified as high risk by current AUA UTUC guidelines, with 86% having high-grade tumors, and 81% having multifocal tumors [ ]. The proportion of patients with pathologic nonmuscle invasive disease was greater in each smoking subgroup when comparing nonsmokers to light CSE, nonsmokers vs. moderate CSE, and nonsmokers to heavy CSE ( Table 1 , P = 0.003 for sub-group comparisons). Perioperative intravesical chemotherapy was utilized in 38% of the entire cohort, with lowest rates in light CSE (20.8%, P = 0.001). Overall utilization of NAC and AC were 8.8% and 20.7% respectively, with no difference across cohorts. There was no difference in history of bladder cancer across cohorts.
| Total N = 1,041 | Nonsmoker N = 586 | Light smoker N = 77 | Moderate smoker N = 170 | Heavy smoker N = 208 | P value | |
|---|---|---|---|---|---|---|
| Years smoking, median (IQR) | 0.0 (0.0–25.0) | 0.0 (0.0–0.0) | 4.0 (2.0–10.0) | 25.5 (20.0–40.0) | 30.0 (25.0–40.0) | <0.001 |
| Packs per day, median (IQR) | 0.0 (0.0–0.5) | 0.0 (0.0–0.0) | 0.2 (0.1–0.5) | 0.5 (0.5–0.8) | 1.0 (1.0–1.5) | <0.001 |
| Pack years, median (IQR) | 0.0 (0.0–15.0) | 0.0 (0.0–0.0) | 2.0 (1.0–5.0) | 13.0 (10.0–20.0) | 40.0 (30.0–53.0) | <0.001 |
| Smoking status, n (%) | <0.001 | |||||
| Nonsmoker | 586 (56.3%) | 586 (100.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Current smoker | 216 (20.7%) | 0 (0.0%) | 40 (51.9%) | 74 (43.5%) | 102 (49.0%) | |
| Former smoker | 239 (23.0%) | 0 (0.0%) | 37 (48.1%) | 96 (56.5%) | 106 (51.0%) | |
| Age, median (IQR) | 71.0 (64.0–78.0) | 72.0 (64.0–79.0) | 69.0 (60.0–77.0) | 72.0 (66.0–77.0) | 69.5 (61.3–75.5) | 0.014 |
| Sex, n (%) | <0.001 | |||||
| Male | 657 (63.2%) | 320 (54.7%) | 46 (59.7%) | 126 (74.1%) | 165 (79.3%) | |
| Female | 383 (36.8%) | 265 (45.3%) | 31 (40.3%) | 44 (25.9%) | 43 (20.7%) | |
| Race, n (%) | <0.001 | |||||
| White | 458 (44.0%) | 197 (33.7%) | 34 (44.2%) | 111 (65.3%) | 116 (55.8%) | |
| Black | 58 (5.6%) | 16 (2.7%) | 22 (28.6%) | 12 (7.1%) | 8 (3.8%) | |
| Hispanic | 78 (7.5%) | 47 (8.0%) | 14 (18.2%) | 8 (4.7%) | 9 (4.3%) | |
| Asian | 405 (38.9%) | 298 (50.9%) | 6 (7.8%) | 34 (20.0%) | 67 (32.2%) | |
| Other | 41 (3.9%) | 27 (4.6%) | 1 (1.3%) | 5 (2.9%) | 8 (3.8%) | |
| ECOG status, n (%) | 0.080 | |||||
| 0 | 519 (53.5%) | 317 (56.5%) | 37 (50.0%) | 69 (44.5%) | 96 (53.0%) | |
| 1 | 392 (40.4%) | 212 (37.8%) | 33 (44.6%) | 70 (45.2%) | 77 (42.5%) | |
| 2 | 60 (6.2%) | 32 (5.7%) | 4 (5.4%) | 16 (10.3%) | 8 (4.4%) | |
| ASA score, n (%) | <0.001 | |||||
| 0–1 | 48 (4.6%) | 12 (2.1%) | 34 (44.2%) | 1 (0.5%) | 1 (0.6%) | |
| 2 | 457 (44.1%) | 294 (50.4%) | 25 (32.5%) | 82 (39.6%) | 56 (33.1%) | |
| 3–4 | 531 (51.3%) | 277 (47.5%) | 18 (23.4%) | 124 (59.9%) | 112 (66.3%) | |
| History of hypertension, n (%) | 617 (61.2%) | 345 (58.9%) | 24 (54.5%) | 114 (67.1%) | 134 (64.4%) | 0.14 |
| History of diabetes, n (%) | 185 (18.4%) | 100 (17.1%) | 4 (9.1%) | 34 (20.0%) | 47 (22.6%) | 0.11 |
| CKD stage, n (%) | 0.50 | |||||
| Stage 0–1 | 134 (14.8%) | 81 (14.9%) | 6 (20.7%) | 20 (14.4%) | 27 (13.8%) | |
| Stage 2 | 343 (37.8%) | 212 (38.9%) | 11 (37.9%) | 41 (29.5%) | 79 (40.5%) | |
| Stage 3 | 393 (43.3%) | 231 (42.4%) | 12 (41.4%) | 71 (51.1%) | 79 (40.5%) | |
| Stage 4–5 | 38 (4.2%) | 21 (3.9%) | 0 (0.0%) | 7 (5.0%) | 10 (5.1%) | |
| History of prior TURBT, n (%) | 180 (18.5%) | 94 (16.4%) | 10 (23.3%) | 40 (25.0%) | 36 (18.4%) | 0.079 |
| Preop. hydronephrosis, n (%) | 558 (53.8%) | 319 (54.6%) | 40 (51.9%) | 91 (54.2%) | 108 (51.9%) | 0.9 |
| AUA risk stratification, n (%) | 0.29 | |||||
| Low risk | 42 (4.9%) | 19 (4.0%) | 5 (9.3%) | 7 (4.6%) | 11 (6.1%) | |
| High risk | 823 (95.1%) | 461 (96.0%) | 49 (90.7%) | 144 (95.4%) | 169 (93.9%) | |
| Presence of multifocal tumor, n (%) | 755 (81.4%) | 439 (83.0%) | 51 (83.6%) | 110 (71.9%) | 155 (84.2%) | 0.011 |
| Tumor location, n (%) | 0.002 | |||||
| Renal pelvis/calyx | 471 (45.4%) | 272 (46.6%) | 23 (29.9%) | 77 (45.6%) | 99 (47.8%) | |
| Ureter | 418 (40.3%) | 245 (42.0%) | 40 (51.9%) | 55 (32.5%) | 78 (37.7%) | |
| Multiple sites | 148 (14.3%) | 67 (11.5%) | 14 (18.2%) | 37 (21.9%) | 30 (14.5%) | |
| Clinical tumor size (cm), median (IQR) | 3.0 (2.0–4.2) | 2.8 (2.0–4.0) | 4.5 (2.7–6.0) | 3.0 (2.0–4.0) | 2.9 (2.0–4.0) | <0.001 |
| Clinical T stage, n (%) | 0.066 | |||||
| cTis, cTa, cT1 | 416 (60.5) | 196 (57.1) | 39 (57.4) | 83 (61.5) | 98 (69.0) | |
| cT2 | 156 (22.7) | 85 (24.8) | 21 (30.9) | 24 (17.8) | 26 (18.3) | |
| cT3, cT4 | 116 (16.9) | 62 (18.1) | 8 (11.8) | 28 (20.7) | 18 (12.7) | |
| Clinical N stage, n (%) | 0.041 | |||||
| cN0 | 940 (91.3) | 544 (93.5) | 67 (88.2) | 149 (88.7) | 180 (88.2) | |
| cN1 | 48 (4.7) | 20 (3.4) | 7 (9.2) | 11 (6.6) | 10 (4.9) | |
| cN2–N3 | 42 (4.1) | 18 (3.1) | 2 (2.6) | 8 (4.8) | 14 (6.9) | |
| Pathologic tumor size (cm), median (IQR) | 3.0 (2.0–4.8) | 3.0 (2.0–4.2) | 5.0 (2.5–6.0) | 3.4 (2.2–4.8) | 3.5 (2.3–5.0) | <0.001 |
| Pathologic T stage, n (%) | 0.002 | |||||
| pT0, pTa, pTis, pT1 | 462 (44.4%) | 233 (39.8%) | 44 (57.1%) | 86 (50.6%) | 99 (47.6%) | |
| pT2 | 188 (18.1%) | 119 (20.3%) | 16 (20.8%) | 24 (14.1%) | 29 (13.9%) | |
| pT3 | 350 (33.6%) | 217 (37.0%) | 14 (18.2%) | 53 (31.2%) | 66 (31.7%) | |
| pT4 | 41 (3.9%) | 17 (2.9%) | 3 (3.9%) | 7 (4.1%) | 14 (6.7%) | |
| Pathologic grade, n (%) | <0.001 | |||||
| Low grade | 144 (14.4%) | 62 (10.9%) | 29 (38.2%) | 27 (16.5%) | 26 (13.8%) | |
| High grade | 854 (85.6%) | 507 (89.1%) | 47 (61.8%) | 137 (83.5%) | 163 (86.2%) | |
| Pathologic N stage, n (%) | 0.070 | |||||
| pN0 | 338 (32.5%) | 182 (31.1%) | 19 (24.7%) | 67 (39.4%) | 70 (33.7%) | |
| pN+ | 79 (7.6%) | 41 (7.0%) | 6 (7.8%) | 9 (5.3%) | 23 (11.1%) | |
| pNx | 624 (59.9%) | 363 (61.9%) | 52 (67.5%) | 94 (55.3%) | 115 (55.3%) | |
| Lymphovascular invasion, n (%) | 171 (17.6%) | 92 (17.0%) | 14 (18.2%) | 32 (20.1%) | 33 (17.1%) | 0.83 |
| Presence of tumor necrosis, n (%) | 251 (26.2%) | 152 (28.9%) | 21 (27.3%) | 29 (18.1%) | 49 (25.3%) | 0.057 |
| Neoadjuvant therapy, n (%) | 0.061 | |||||
| None | 949 (91.2%) | 546 (93.2%) | 73 (94.8%) | 147 (86.5%) | 183 (88.0%) | |
| Noncisplatin-based therapy | 14 (1.3%) | 6 (1.0%) | 0 (0.0%) | 4 (2.4%) | 4 (1.9%) | |
| Cisplatin-based therapy | 78 (7.5%) | 34 (5.8%) | 4 (5.2%) | 19 (11.2%) | 21 (10.1%) | |
| Adjuvant therapy, n (%) | 0.70 | |||||
| None | 700 (79.3%) | 388 (78.7%) | 64 (86.5%) | 118 (77.6%) | 130 (79.3%) | |
| Nonplatinum adjuvant therapy | 101 (11.4%) | 61 (12.4%) | 4 (5.4%) | 18 (11.8%) | 18 (11.0%) | |
| Platinum-based adjuvant therapy | 82 (9.3%) | 44 (8.9%) | 6 (8.1%) | 16 (10.5%) | 16 (9.8%) | |
| Bladder cuff management, n (%) | 0.76 | |||||
| Bladder cuff not removed | 18 (1.8%) | 8 (1.4%) | 2 (2.7%) | 4 (2.4%) | 4 (2.1%) | |
| Bladder cuff removed | 974 (98.2%) | 550 (98.6%) | 73 (97.3%) | 160 (97.6%) | 191 (97.9%) | |
| Lymph node dissection, n (%) | 370 (38.2%) | 185 (34.0%) | 30 (40.5%) | 66 (42.0%) | 89 (45.9%) | 0.018 |
| Intravesical chemotherapy, n (%) | 395 (37.9%) | 237 (40.4%) | 16 (20.8%) | 74 (43.5%) | 68 (32.7%) | 0.001 |
| Prior history of bladder cancer, n (%) * | 157 (24.8%) | 78 (25.0%) | 8 (21.1%) | 33 (22.8%) | 38 (27.7%) | 0.77 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree