Highlights
- •
Segmental ureterectomy was feasible for selected ureteral cancer patients.
- •
Segmental ureterectomy faced a considerable risk of local recurrence and intravesical recurrence.
- •
G3 and lymphatic vascular infiltration were independent risk factors for local recurrence.
- •
Male, sarcomatoid differentiation and positive surgical margin were independent risk factors for intravesical recurrence.
Abstract
Objective
To investigate the pattern and risk factors of local recurrence and intravesical recurrence of ureteral upper tract urothelial carcinoma (UTUC) following segmental ureterectomy (SU).
Methods
From February 2012 to August 2021, a retrospective analysis was conducted on patients following SU. Univariate and multivariate Cox regression analysis were used to evaluate the risk factors. Kaplan–Meier curves were employed to illustrate survival outcomes.
Results
Among 88 patients, 50 (57%) were male, with a median age of 71 (IQR: 62–77) years. The procedures of ureteral reconstruction included ureteral reimplantation in 77 (88%) cases, ureteroureteral anastomosis in 9 (10 %) cases, Boari flap ureteroplasty with psoas hitch in 1 (1%) case, and cutaneous ureterostomy in 1 (1%) case. The median follow-up time was 44.5 months. The 3-year rate of local recurrence, lymph node metastasis, ipsilateral upper urinary tract recurrence and intravesical recurrence was 31.6%, 19.0%, 22.2% and 35.7%, respectively. G3 (HR = 3.355, 95% CI 1.375–8.184, P = 0.008), and lymphatic vascular infiltration (HR = 3.127, 95% CI 1.043–9.373, P = 0.042) were independent risk factors for local recurrence. G3 (HR = 3.782, 95% CI 1.036–13.812, P = 0.044) was an independent risk factor for lymph node metastasis. Sarcomatoid differentiation (HR = 3.943, 95% CI 1.087–14.308, P = 0.037) was an independent risk factor for ipsilateral upper urinary tract recurrence. Previous or concurrent bladder cancer (HR = 3.280, 95% CI 1.667–6.453, P = 0.001) and sarcomatoid differentiation (HR = 4.442, 95% CI 1.317–14.989, P = 0.016) were independent risk factor for intravesical recurrence. The most common regions for bladder recurrence were posterior wall (21%), same lateral wall (16%) and trigon (16%).
Conclusion
SU is a feasible treatment for selected UTUC patients, yet it is associated with a considerable risk of local and intravesical recurrence. Careful monitoring and active adjuvant therapy are essential to minimize the recurrence rate for patients with risk factors.
1
Introduction
Radical nephroureterectomy (RNU) is the standard treatment for upper tract urothelial carcinoma (UTUC) [ ]. However, RNU can lead to reduced renal function, potentially compromising eligibility for cisplatin-based chemotherapy [ ], and may increase the risk of postoperative recurrence [ ]. Consequently, kidney-sparing surgery (KSS) has become a preferred option in selected patients, as it provides better preservation of renal function and enhances tolerance to subsequent therapies.
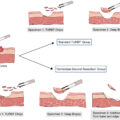
Segmental ureterectomy (SU) is one of the KSSs for ureter carcinoma, and appears to be associated with a median estimated glomerular filtration rate (eGFR) decrease of only 1 ml/min/1.73 m 2 at 3 months postoperatively compared to 15 ml/min/1.73 m 2 after RNU [ ]. In selected patients, SU has demonstrated comparable oncological outcomes to RNU with better renal function preservation [ , ]. According to European Association of Urology (EAU) guideline, SU is only recommended for patients with low-risk disease [ ]. However, American Urological Association (AUA) guidelines suggest that both RNU and SU are appropriate for surgically eligible patients with high-risk UTUC [ ]. Patients with a strong preference for KSS or those with contraindications such as solitary kidney, chronic kidney disease (CKD), and multiple comorbidities, may not be suitable candidates for RNU. For these high-risk patients, SU could serve as a feasible option [ ].
Adjuvant radiotherapy following RNU may reduce the risk of local recurrence and improve cancer specific survival in high-risk UTUC patients [ , ]. Adjuvant radiotherapy administered to regions at high risk of recurrence following SU may reduce the risk of local recurrence and intravesical recurrence, potentially achieving oncological outcomes comparable to RNU. However, the incidence and pattern of local recurrence and intravesical recurrence following SU warrant further investigation. In this study, we explored the pattern and risk factors associated with local recurrence and intravesical recurrence after SU for UTUC.
2
Methods
2.1
Patients
From February 2012 to August 2021, all patients with ureteral cancer after SU in our hospital were retrospectively reviewed. The exclusion criteria were as follows: (1) history of adjuvant radiotherapy or systemic medication therapy (n = 8); (2) history of other malignancy besides urothelial carcinoma (n = 5); (3) follow-up period less than 6 months (n = 4).
For patients with concomitant bladder tumors, transurethral resection of bladder tumors was performed concurrently with SU. Lymph node dissection was conducted when lymph node metastasis was suspected according to the preoperative evaluation or enlarged lymph nodes were detected intraoperatively. The manner of ureteral reconstruction after SU was determined by the location and length of the lesion. The pathological staging was based on TNM 2017 classification for UTUC [ ]. Tumor grade was determined according to the World Health Organization (WHO) classification of 1973 [ ]. The risk stratification was based on EAU guideline [ ].
Patient records were anonymized and deidentified before analysis. The retrospective data collection and analysis procedures were approved by the Ethics Committee of our hospital (2023-SR-162-002), waiving the need for written informed consent.
2.2
Follow-up
None of patients received neoadjuvant systemic treatments. All patients received a single dose of intravesical therapy within one week after SU. Patients were followed every 3 months for the first 2 years and every 6 months within 2-5 years. Subsequently, follow-up intervals were extended to annually for patients without recurrence concerns. Cystoscopy, chest X-rays and abdominal ultrasound, CT, and/or MRI were assessed. 18 F- FDG PET/CT and ureteroscopy were employed when identifying recurrence was difficult.
Local recurrence was defined as abdominal and pelvic recurrence, including lymph node drainage area recurrence and ipsilateral upper urinary tract recurrence. The adenopathy definition was defined by a short axis diameter of 1 cm or larger in CT and/or MRI images, and/or PET/CT visualization of hypermetabolic activity regardless of size [ ]. Local recurrence was reviewed and confirmed independently by 2 senior radiological physicians. The presence of intravesical recurrence and its location were assessed through cystoscopy with pathologic confirmation [ ]. The first local recurrence and intravesical recurrence were recorded in this study for analysis.
2.3
Statistics
Continuous variables not normally distribution were presented as medians (first to third quartile, Q1–Q3), and categorical variables as numbers (percentages). Univariate and multivariate Cox regression analysis were performed to evaluate the risk factors of local recurrence, lymph node metastasis, ipsilateral upper urinary tract recurrence and intravesical recurrence. Kaplan-Meier curves were employed to illustrate survival outcomes, while the log-rank test was used to evaluate differences in survival among subgroups. Statistical analyses were executed using SPSS 26.0 software (SPSS Software Inc., Chicago, IL, USA). A P -value < 0.05 was considered statistically significant.
3
Results
As shown in Table 1 , a total of 88 patients were involved in this study, including 50 (56.8%) males, with a median age of 71 (IQR: 62–77) years. Six (7%) patients presented with concomitant bladder cancer, and 25 (28%) patients had a history of bladder cancer prior to UTUC. Among the 14 patients who underwent lymph node dissection, 3 (21.4%) had positive lymph nodes. According to EAU stratification, 71 (81%) cases were classified as high-risk. The procedures of ureteral reconstruction included ureteral reimplantation in 77 (88%) cases, ureteroureteral anastomosis in 9 (10 %) cases, Boari flap ureteroplasty with psoas hitch in 1 (1%) case, and cutaneous ureterostomy in 1 (1%) case. The median follow-up period was 44.5 (IQR: 33–68) months.
| Variables | Number (%) |
|---|---|
| Age (years), median (Q1–Q3) | 71 (62–77) |
| Gender, n (%) | |
| Male | 50 (57) |
| Female | 38 (43) |
| Concomitant bladder cancer, n (%) | |
| Present | 82 (93) |
| Absent | 6 (7) |
| Prior history of bladder cancer, n (%) | |
| Present | 25 (28) |
| Absent | 63 (72) |
| Diseased side, n (%) | |
| Left | 44 (50) |
| Right | 44 (50) |
| Location of tumor, n (%) | |
| Proximal | 3 (3) |
| Middle | 2 (2) |
| Distal | 83 (95) |
| Diameter of tumor (cm), median (Q1-Q3) | 2.0 (1.5–2.5) |
| Tumor stage, n (%) | |
| Tis/Ta | 4 (4) |
| T1 | 37 (42) |
| T2 | 35 (40) |
| T3 | 12 (14) |
| WHO Grade, n (%) | |
| G1 | 4 (4) |
| G2 | 43 (49) |
| G3 | 41 (47) |
| Lymph node stutus | |
| Negative | 11 (12) |
| Positive | 3 (3) |
| Nx | 74 (85) |
| EAU risk stratification, n (%) | |
| High-risk | 71 (81) |
| Low-risk | 17 (19) |
| Positive surgical margin, n (%) | 8 (9) |
| Histological differentiation, n (%) | |
| Squamous cell differentiation | 6 (7) |
| Glandular differentiation | 5 (6) |
| Sarcomatoid differentiation | 4 (4) |
| Lymphatic vascular infiltration, n (%) | 9 (10) |
Local recurrence was observed in 34 (39%) cases, with 20 (23%) cases experienced lymph node metastasis, and 22 (25%) cases underwent ipsilateral upper urinary tract recurrence. Six patients underwent RNU after SU due to ipsilateral upper urinary tract recurrence. Intravesical recurrence was observed in 39 patients (44%), including 24 cases with multisite intravesical recurrence. Five patients underwent radical cystectomy due to intravesical recurrence. The 3-year rate of local recurrence, lymph node metastasis, ipsilateral upper urinary tract recurrence and intravesical recurrence was 31.6%, 19.0%, 22.2% and 35.7%, respectively.
Univariate Cox regression analysis of local recurrence, lymph node metastasis and ipsilateral upper urinary tract recurrence were presented in Table 2 . The risk factors for local recurrence comprised T2-T3, G3, sarcomatoid differentiation and lymphatic vascular infiltration (LVI); for lymph node metastasis, they comprised T2-T3 and G3; for ipsilateral upper urinary tract recurrence, they comprised G3 and sarcomatoid differentiation .Multivariate Cox regression analysis demonstrated that G3 (HR = 3.355, 95% CI 1.375–8.184, P = 0.008), and lymphatic vascular infiltration (HR = 3.127, 95% CI 1.043-9.373, P = 0.042) were independent risk factors for local recurrence. G3 (HR = 3.782, 95% CI 1.036-13.812, P = 0.044) was an independent risk factor for lymph node metastasis. Sarcomatoid differentiation (HR = 3.943, 95% CI 1.087-14.308, P = 0.037) was an independent risk factor for ipsilateral upper urinary tract recurrence ( Table 3 ). The 3-year local recurrence-free survival (LRFS) rate of LVI group and no-LVI group was 33.3% vs. 72.4%, respectively ( P = 0.001) ( Fig. 1A ). The 3-year LRFS rate of G3 group and G1-G2 group was 47.6% vs. 85.1% respectively ( P < 0.001) ( Fig. 1B ). The 3-year lymph node metastasis-free survival rate of G3 group and G1-G2 group was 63.3% vs. 95.6%, respectively ( P = 0.001) ( Fig. 1C ). The 3-year ipsilateral upper urinary tract recurrence-free survival rate of sarcomatoid differentiation group and no sarcomatoid differentiation group was 25.0% vs. 82.2%, respectively ( P = 0.001) ( Fig. 1D ).
| Variables | Local recurrence | Lymph node metastasis | Ipsilateral upper urinary tract recurrence | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P -value | HR (95% CI) | P -value | HR (95% CI) | P -value | |
| Age (≥70 years/<70 years) | 0.805(0.406–1.594) | 0.533 | 0.92(0.374–2.267) | 0.857 | 0.826(0.358–1.906) | 0.654 |
| Gender (Male/Female) | 1.078(0.539–2.154) | 0.832 | 2.402(0.861–6.703) | 0.094 | 0.813(0.35–1.89) | 0.631 |
| Previous or concurrent bladder cancer (Presence/ Absence) | 1.509(0.748–3.044) | 0.250 | 0.774(0.279–2.152) | 0.624 | 2.053(0.881–4.784) | 0.095 |
| Laterality (Right/ Left) | 0.818(0.413–1.62) | 0.564 | 1.209(0.486–3.011) | 0.683 | 0.755(0.326–1.75) | 0.513 |
| Diameter of tumor (≥2cm/ <2cm) | 1.185(0.597–2.352) | 0.628 | 0.894(0.363–2.202) | 0.808 | 2.18(0.888–5.352) | 0.089 |
| Tumor stage (T2–T3/ Tis-T1) | 2.272(1.096–4.707) | 0.027 | 0.229(0.075–0.692) | 0.009 | 0.752(0.322–1.757) | 0.510 |
| WHO Grade (G3/ G1–G2) | 3.672(1.738–7.76) | 0.001 | 5.649(1.868–17.083) | 0.002 | 2.638(1.099–6.332) | 0.030 |
| EAU risk stratification (High-risk/ Low-risk) | 3.001(0.915–9.842) | 0.070 | 5.499(0.733–41.265) | 0.097 | 1.887(0.557–6.39) | 0.308 |
| Squamous differentiation (Presence/ Absence) | 1.294(0.394–4.243) | 0.671 | 0.769(0.103–5.773) | 0.799 | 2.048(0.604–6.941) | 0.250 |
| Glandular differentiation (Presence/ Absence) | 1.819(0.554–5.976) | 0.324 | 1.692(0.39–7.338) | 0.483 | 1.82(0.424–7.807) | 0.420 |
| Sarcomatoid differentiation (Presence/ Absence) | 3.81(1.151–12.615) | 0.029 | 1.256(0.167–9.42) | 0.825 | 5.911(1.722–20.292) | 0.005 |
| Lymphatic vascular infiltration (Presence/ Absence) | 3.803(1.634–8.85) | 0.002 | 2.981(0.986–9.011) | 0.053 | 2.682 (0.899–8.002) | 0.077 |
| Positive surgical margin (Presence/ Absence) | 2.276(0.875–5.92) | 0.092 | 2.517(0.73–8.679) | 0.144 | 2.873 (0.966–8.544) | 0.058 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree