Highlights
- •
The standard prostate biopsy method results in overdiagnosis and overtreatment.
- •
ips-SB+TB can be an alternative for detecting prostate cancer (PCa).
- •
Ips-SB+TB has comparable efficiency in detecting clinically significant PCa.
- •
Ips-SB+TB can reduce biopsy cores and overdiagnosis.
Abstract
Background
The current standard prostate biopsy method, which combine systematic biopsy (SB) with targeted biopsy (TB), has shortcomings such as overdiagnosis and overtreatment. To evaluate the effectiveness of ipsilateral systematic biopsy (ips-SB) combined with targeted biopsy (ips-SB+TB) and contralateral SB (con-SB) combined with TB (con-SB+TB) as potential alternatives to SB+TB.
Methods
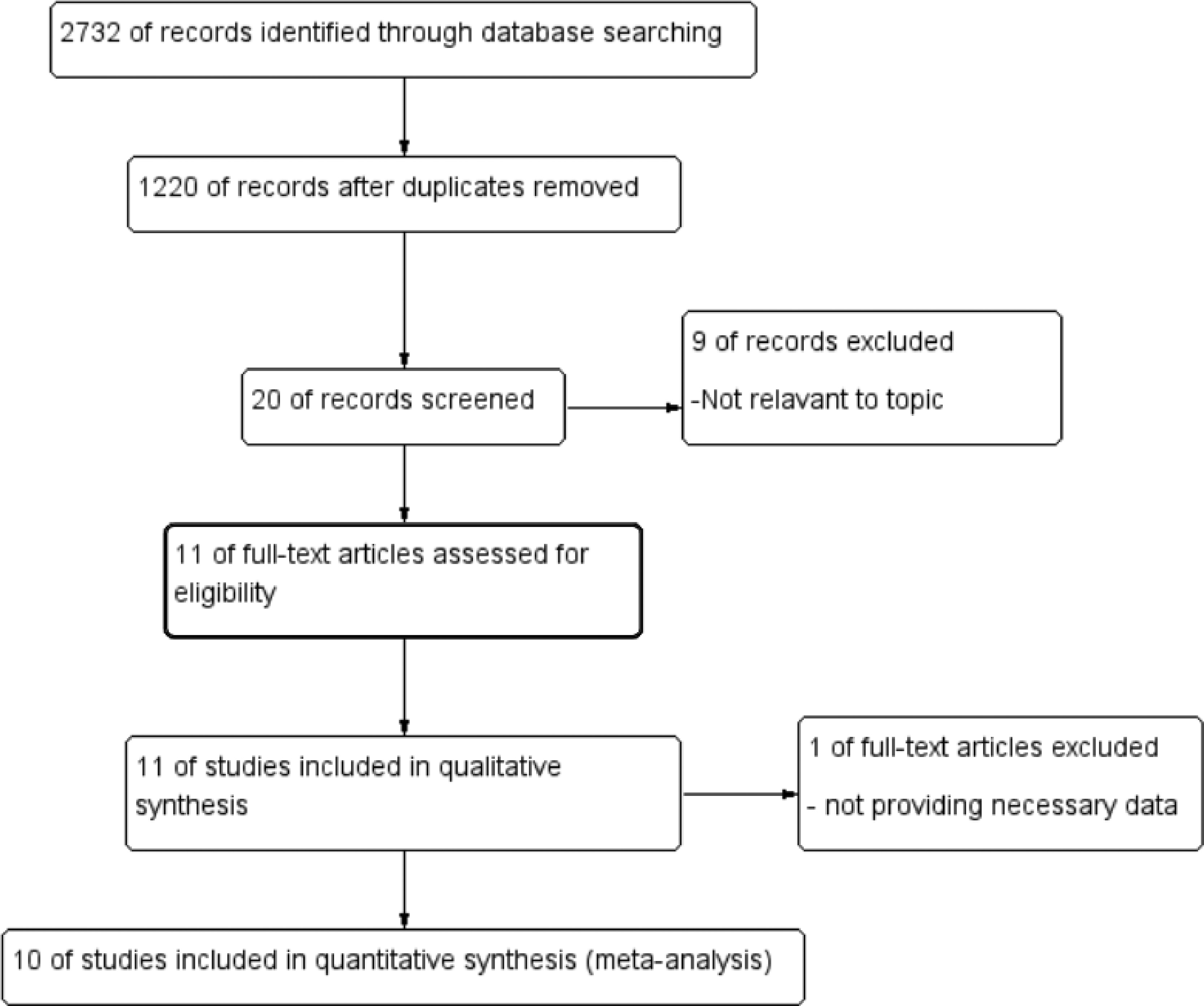
A comprehensive literature search was conducted in Cochrane, Embase, Ovid, and PubMed databases until September 2024. 2,732 references were identified, and 11 records were included.
Main findings
The study included a total of 5,249 patients and revealed that ips-SB+TB detected slightly less PCa than SB+TB with a relative risk (RR) of 0.95 (95% CI 0.91, 1.00), P = 0.05. In terms of csPCa detection, ips-SB+TB showed a comparable detection rate with SB+TB (RR 0.98 [95% CI 0.94, 1.01], P = 0.60). There was a statistically significant difference in csPCa detection between con-SB+TB and SB+TB (RR 0.92 [95% CI 0.86, 0.99], P = 0.02). The detection rates of clinically insignificant PCa (ciPCa) were comparable between con-SB+TB vs. SB+TB (con-SB+TB vs. SB+TB: RR 0.90 [95% CI 0.79, 1.04], P = 0.15). However, fewer ciPCa cases were detected in ips-SB+TB compared to SB+TB (RR 0.86 [95% CI 0.75, 0.99], P = 0.04).
Conclusions
In this review, our analysis highlights ips-SB+TB has the comparable detection efficiency of PCa and csPCa compared to SB+TB, and its potential to be the substitute of the SB+TB with less cores and less detection of ciPCa.
1
Introduction
Early detection is crucial for effective treatment of prostate cancer (PCa), the second most commonly diagnosed cancer in men [ ]. The advent of multi-parametric magnetic resonance imaging (mpMRI) has fundamentally changed the diagnosis of PCa, playing a role in the detection and localization of PCa and apparently improving the detection rate of clinically significant PCa (csPCa) [ , ]. However, due to considerable overlap between the appearances of benign and malignant lesions in imaging, PCa is diagnosed by blindly puncturing the entire organ, rather than just the identified lesion by imaging [ ]. Currently, MRI/ultrasound (US) fusion TB is considered to enhances the detection of csPCa, while reducing the detection of clinically insignificant PCa (ciPCa) [ , , ]. When TB combined with SB, a higher detection rate of PCa is achieved, and both techniques have shown significant added value [ , , ].
In the EAU guidelines 2023, The combination of TB and SB (SB+TB) is strongly recommended when MRI is positive (i.e., PI-RADS > 3). SB+TB, as the most accurate biopsy scheme, has been validated by a few studies for its necessity and superiority in detection rate, Pathologic concordance, and risk of upgrading compared with SB and TB respectively [ , ]. However, the increase of the biopsy cores resulting in higher incidence of complications, higher biopsy expenses and more detection of ciPCa which may lead to overdiagnosis and overtreatment [ , ]. Exploring and refining the biopsy technique to attain a detection rate of csPCa with fewer cores is of utmost importance.
Recently, several studies have investigated and highlighted the potential of regional biopsy combined TB regimens including ipsilateral SB (ips-SB) combined TB (ips-SB+TB) and contralateral SB (con-SB) combined TB (con-SB+TB) as the substitutes of SB+TB, revealing that ips-SB+TB increases csPCa detection and decreasing ciPCa detection [ , ]. Furthermore, few studies have suggested that distant systematic biopsy cores have limited impact in detecting csPCa, and it is recommended that SB should only be performed on the ipsilateral side of the target lesion identified on MRI [ , , ]. Therefore, this study was conducted to assess the detection rate of csPCa using different prostate sampling methods and to confirm whether ips-SB+TB or con-SB+TB could yield similar csPCa detection rates as SB+TB.
2
Materials and methods
The protocol has been registered in the International Prospective Register of Systematic Reviews database (PROSPERO: CRD42023478032).
2.1
Search strategy
We conducted a comprehensive literature search across multiple databases, including Embase, PubMed, Ovid, and the Cochrane Library, using the following search terms (not limited to): “prostate cancer,” “targeted biopsy,” and “systematic biopsy.” We refined our search using boolean operators AND and OR. We employed Medical Subject Heading phrases “prostatic neoplasms” and “magnetic resonance imaging.” We screened all potentially eligible research by reading the full text. Additionally, we evaluated citation and reference lists in papers to identify relevant studies.
2.2
Inclusion and exclusion criteria
Studies were considered if they satisfied all of the following requirements: (1) Patients with elevated levels of prostate-specific antigen (PSA), whether or not they have had a previous biopsy underwent mpMRI; (2) At least one unilateral MRI lesion was detected; (3) SB+TB was performed in the same patient, or unilateral SB combined with TB (ips-SB+TB or con-SB+TB) performed separately in different groups with comparable baseline characteristics; (4) The required data pertaining to the detection rate of PCa and csPCa could be extracted from both the paper and its supplementary materials ; and (5) Studies written in a language other than English, meeting abstracts, and reviews were not included. Phantom and animal studies were also excluded.
2.3
Data extraction
Two reviewers conducted a comprehensive search of established databases to screen all abstracts and titles. Duplicate data was removed using ENDNOTE software. Two reviewers conducted a comprehensive search of established databases to screen all abstracts and titles. Upon selecting papers, a thorough full-text review was conducted, which resulted in their subsequent rejection for various reasons. Any papers that were included underwent reference list analysis, and additional relevant articles were identified.
Study characteristics such as publication year, country, sample size, and type of study were recorded, along with MRI protocol details including field strength, scoring system, and sequence. Basic patient features like age, prostate volume, PSA, and Digital Rectal Exam were also noted, along with relevant biopsy information such as biopsy pattern, fusion technique, targeted fusion system, PCa/csPCa detection details, and reference standard definition of csPCa, all of which were extracted independently.
2.4
Statistical analysis
We utilized RevMan 5.4 software (Cochrane Collaboration, London, UK) and R software (R version 4.2.2) with the package Meta for statistical analysis. In order to create summary estimates, either a fixed effects model ( P > 0.1 and I 2 < 50%) or a random effects model ( P < 0.1 or I 2 > 50%) was used, depending on whether or not there was heterogeneity.
PCa, ciPCa, and csPCa detection rates for different biopsy schemes were expressed using the Inverse Variance method as a risk ratio (RR) with 95% confidence intervals (CI). Statistical significance was determined by P values of 0.05 or less. The analysis of statistical heterogeneity utilized a chi-squared test with N-1 degrees of freedom, an I2 test, and an alpha value of 0.1. I 2 values corresponding to low (25%), medium (50%) and high (75%) degrees of heterogeneity were observed. Begg’s funnel plot and Peters’ test were utilized to statistically assess the asymmetry of the funnel plot that is linked with publication bias. Subgroup analysis was performed according to the number of unilateral intraprostatic lesions in the included patients.
2.5
Quality assessment
The study utilized the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) and QUADAS Comparative (QUADAS-C) [ , ]. The QUADAS-2 tool comprises 4 distinct domains: patient selection, index test, reference standard, and flow and timing. Risk of bias assessment is conducted for each domain, while concerns about applicability are assessed for the first 3 domains. The QUADAS-C tool was created as an extension of QUADAS-2, adding extra questions to each domain, to evaluate bias risk in comparative diagnostic accuracy studies.
3
Result
3.1
Study selection and characteristics
From the literature search, our study identified a total of 2,732 references. We comprehensively assessed 1,512 references by analyzing their titles and abstracts, and further examined 20 records by reading the full text ( Fig. 1 ). Ultimately, 11 records met our inclusion criteria and were included in the analysis, with a total of 5249 patients analyzed in our study. In Table 1 and Supplementary Table 1 , we displayed the characteristics of the included research. Elven studies were included in the qualitative synthesis [ , , ], while 10 studies were included in the quantitative synthesis [ , , ]. One study did not utilize the Prostate Imaging-Reporting and Data System (PI-RADS) [ ]. Another 2 studiesapplied both PI-RADS v2 and v2.1 [ , ]. Four studies specifically utilized PI‐RADS v2 [ , , , ], while the remaining 4 studies employed PI‐RADS v2.1 [ , , , ]. Nine studies performed targeted prostate biopsies through the transrectal route [ , , , , ]. While 1 study adopted the transperineal approach, and another 1 adopted the both 2 approaches [ , ]. The definitions of csPCa and ciPCa were consistent across all studies [ , , ]. CsPCa was defined as ISUP GG ≥ 2 (or Gleason > 6), while ciPCa was defined as ISUP GG = 1 (or Gleason = 6). However, the study by Wang et al. additionally reported the Epstein criteria, which incorporate a Gleason score (GS) > 6 or GS 6 with ≥ 50% of cancer per core involvement, or > 2 cores with cancer [ ]. Three studies included patients with a single unilateral MRI lesion [ , , ]. And 7 studies enrolled patients with unilateral intraprostatic lesions, either singular or multiple [ , , , ]. Pertinent information was not provided by just 1 study [ ]. The biopsy pattern, the detection rate of PCa, the parameter of the MRI and other relevant characteristics are summarized in Table 1 and Supplementary Table 1 .
| Study (Year) | Country | Type of study | Sample size (n) | Prostate volume, ml | PSA (ng/ml) | Age (yr) | Field Strength | Sequence | MRI Scoring System (Positive MRI) | Targeted Fusion System | Biopsy fusion technique | Targeted Biopsy route |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bryk (2016) | USA | retrospective | 211 | NA | 5.3 (3.8-6.9) a | 61.0 (56-66) a | 3.0T | T2W/DWI | No | Artemis prostate biopsy system and ProFuse (Eigen, Grass Valley, CA) software for MRI segmentation | software | Transrectal |
| Yusim (2023) | Israe | retrospective | 364 | 51 (36.0–70.7) a | 6.6 (5.0–9.0) a | 69 (65–73) a | 3.0T | T2W/DWI/DCE | PI-RADS v2.1 | BioJet Target, Release 3.0 | software | Transperineal |
| Phelps (2023) | USA | retrospective | 212 | 62.5 (44–88.3) a | 6.48 (4.30–9.83) a | 67 (61–72) a | 3.0T | T2W/DWI/ADC/DCE | PI-RADS v2.1 1-5 | UroNav; Invivo, Gaines ville, Florida | NA | Transrectal |
| Ruan (2023) | China | prospective and retrospective | 464 | 45.98 (33.05–64.02) a | 9.05 (6.52–12.46) a | 67.36 ± 8.03 b | 3.0T | T2W/DWI/ADC/DCE | PI-RADS v2.1 | NA | NA | Transperineal |
| Freifeld (2018) | USA | retrospective | 116 | 54.12 ± 30.39 b | 10.36 ± 14.59 b | 63.7 ± 8.33 b | 3.0T | T2W/DWI/DCE | PI-RADS v2 | UroStation, Koelis; UroNav InVivo | NA | Transrectal |
| Wang (2021) | China | prospective | 279 | 57.00 (41.00–82.30) a | 10.04 (6.38–18.00) a | 71 (65–77) a | 3.0T | T2W/DWI/DCE | PI-RADS v2 | Virtual Navigator (Esaote, Genoa, Italy). | software | Transrectal |
| Jager (2022) | Netherlands | retrospective | 228 | 646 (19-73) a | 7.6 (1.72-13.48) a | 65.6 ± 7.7 b | 1.5T or 3.0T | T2W/DWI/DCE | PI‐RADS v2 | ProFuse® (Eigen, Grass Valley, USA) | software | Transrectal/Transperineal |
| Bourgeno (2024) | Belgium | retrospective | 2387 | 48 (36–65) a | 6.9 (5–9.6) a | 68 (62–73) a | 1.5T or 3.0T | T2W/DWI/DCE | PI-RADS v2 and v2.1 | KOELIS, La Tronche, France | software | transrectal or transperineal |
| Shen (2020) | China | retrospective | 113 | 47.73 (32.08-62.95) a | 9.70 (6.65-16.22) a | 70.7 ± 9.14 | 3.0T | T2W/DWI/DCE | PI‐RADS v2 | GE Healthcare, Boston, MA, USA | software | Transrectal |
| Yu (2024) | China | retrospective | 122 | 35.1 (26.5–49.4) a | 9.99 (6.71–19.18) a | 70 (64–76) a | NA | T2W/DWI/DCE | PI‐RADS v2.1 | NA | cognitive | Transrectal |
| Diamand (2022) | Belgium | retrospective | 753 | 45 (34–57) a | 7 (5.2–9.8) a | 66 (62–70) a | 1.5T or 3.0T | T2W/DWI/DCE | PI-RADS v2 or 2.1 | KOELIS, La Tronche, France | software | Transrectal |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree