Highlights
- •
Development of novel therapies is needed for patients with high-risk bladder cancer.
- •
TAR-200 is a gemcitabine intravesical releasing system designed for bladder cancer.
- •
TAR-200 was well tolerated with antitumor activity in phase I bladder cancer trials.
- •
Phase II and III studies of TAR-200 in patients with bladder cancers are ongoing.
- •
Data from SunRISe-1 support study in high-risk non–muscle-invasive bladder cancer.
Abstract
Treatment options for recurrent high-risk non–muscle-invasive bladder cancer (HR NMIBC) and muscle-invasive bladder cancer (MIBC) are limited, highlighting a need for clinically effective, accessible, and better-tolerated alternatives. In this review we examine the clinical development program of TAR-200, a novel targeted releasing system designed to provide sustained intravesical delivery of gemcitabine to address the needs of patients with NMIBC and of those with MIBC. We describe the concept and design of TAR-200 and the clinical development of this gemcitabine intravesical system in the SunRISe portfolio of studies. This includes 3 phase I studies evaluating the safety and initial tumor activity of TAR-200 and 5 phase II/III studies assessing the efficacy and safety of TAR-200, with or without systemic cetrelimab, as a treatment option for patients with HR NMIBC (bacillus Calmette-Guérin naive [papillary and carcinoma in situ] and MIBC (neoadjuvant and patients ineligible for or refusing radical cystectomy). Pharmacokinetics demonstrate intravesical gemcitabine delivery via TAR-200 over a prolonged period without detectable plasma levels. Phase I studies showed that TAR-200 is well tolerated, with preliminary antitumor activity in intermediate-risk NMIBC and MIBC. Preliminary data from the phase IIb SunRISe-1 study demonstrate that TAR-200 monotherapy is safe and effective in patients with bacillus Calmette-Guérin–unresponsive high-risk NMIBC. TAR-200 represents an innovative approach to the local treatment of bladder cancer.
Graphical Abstract
1
Introduction
1.1
Unmet need in bladder cancer
Globally, bladder cancer is the ninth most common cancer, with an estimated 614, 298 new diagnoses and 220, 596 cancer-specific deaths in 2022 [ ]. The estimated 1-year prevalence for bladder cancer was 491, 243 in 2022—the seventh highest across cancers worldwide [ ]. The burden of bladder cancer is also significant, with 54 disability-adjusted life years lost per 100, 000 individuals, making bladder cancer the fifteenth most burdensome cancer globally in 2019 [ ]. Despite this global disease burden, the current standard of care for recurrent high-risk non–muscle-invasive bladder cancer (HR NMIBC) and muscle-invasive bladder cancer (MIBC) offers patients few treatment options. This is due to multiple factors, including varying drug toxicity, quality of life detriments, or inadequate clinical outcomes, thus highlighting a need for clinically effective, accessible, and better-tolerated alternatives. Additionally, bladder cancer is among the most expensive tumors for healthcare systems, as evidenced by the cost of MIBC treatments (e.g., the 5-year median costs for trimodal therapy and radical cystectomy [RC] are approximately US $400, 000 and US $225, 000, respectively) [ , ].
1.2
Contemporary management of HR NMIBC
Treatment options for HR NMIBC include transurethral resection of bladder tumor (TURBT) and subsequent induction and maintenance with intravesical bacillus Calmette-Guérin (BCG), which was approved for bladder cancer by the US Food and Drug Administration (FDA) in the 1990s [ , ]. Compared with BCG as standard induction therapy alone, BCG maintenance therapy was shown to significantly increase recurrence-free survival [ ]. Response rates approach 85 % for HR NMIBC [ ]; however, more than 50 % of the individuals experience recurrence within 4 years after BCG treatment [ ]. Many patients do not complete the maintenance regimen per guideline recommendations due to significant treatment-associated toxicity: approximately two thirds of patients experience local toxicity, and approximately one third suffer systemic toxicity [ , ]. Furthermore, given variable yet substantial challenges with respect to global BCG manufacture shortages, limited supplies for some geographical regions necessitate strategies to provide patients with optimal treatment, including prioritizing high-risk patients for induction or giving split (i.e., 1/3 or 1/2) doses [ ]. For patients with disease that recurs following treatment with adequate BCG, RC is the standard of care recommended by the American Urological Association/Society of Urologic Oncology (AUA/SUO) and European Association of Urology (EAU) guidelines [ , ]; however, in the real-world setting, this treatment option is utilized at variable rates owing to patient preference in this older population with a high burden of frailty and comorbidity [ ].
1.3
Current treatment of MIBC
Treatment options for MIBC include RC with neoadjuvant cisplatin-based therapy for eligible patients [ ]. Approximately 50 % of patients are cisplatin ineligible due to age- and/or disease-related comorbidities [ , , ]. Trimodal therapy, including “maximal” TURBT, radiotherapy, and chemotherapy, is a bladder-sparing option that may be offered to select patients with MIBC, and its use varies by region [ ].
1.4
RC in HR NMIBC and MIBC
Although it is a treatment option for patients with recurrent HR NMIBC following adequate BCG exposure and for patients with MIBC [ , ], RC may be associated with significant complication rates, morbidity, hospital readmissions, and perioperative mortality [ ]. The weighted overall complication rate for the initial hospitalization is approximately 35 %, with postsurgical rates increasing to 39 % at 30 days and 60 % (ranging to >80 %) at 90 days [ ]. Based on recent prospective studies, the 90-day mortality rate from RC ranges between 4.4 % and 6.5 % [ ]. In older patients, 90-day mortality rates of 9 % to 15 % have been reported [ , ]. Patients selecting RC also require urinary diversion and thus will often have other comorbidities, which may impact their health-related quality of life and further affect the morbidity and mortality rates among this population [ ].
2
Concept and design of TAR-200
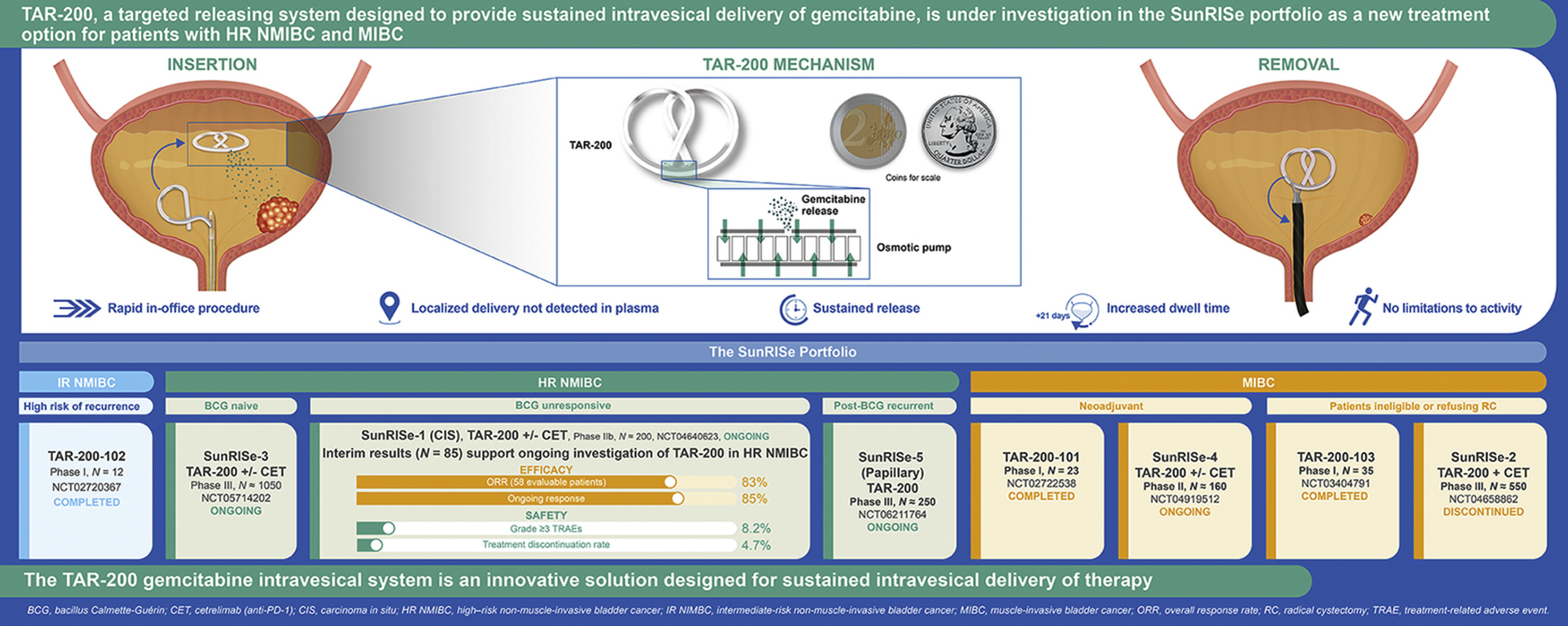
A limitation of the current intravesical delivery of liquid-based therapy for the treatment of localized bladder cancer is the inefficiency related to limited drug exposure, sometimes described as the “dwell” or “contact” time of the drug, to the endothelium (i.e., most of the therapeutic drug is evacuated from the bladder within 1 to 2 h post instillation at the time of the patient’s first void). To treat bladder conditions, a drug delivery system must also overcome challenges related to drug dilution due to continuous urine formation. Theoretically, repeated instillations could mitigate the progressive dilution of intravesical therapy; however, this presents substantial logistical challenges with respect to patient burden and clinic implementation. Thus, the potential to deliver a therapeutically active dose of medication directly into the patient’s bladder in a sustained fashion, while concomitantly avoiding systemic toxicities, would present a highly desirable option for patients with localized disease. The TAR-200 gemcitabine intravesical system was designed to address this critical need for sustained drug delivery within the bladder ( Fig. 1 ).
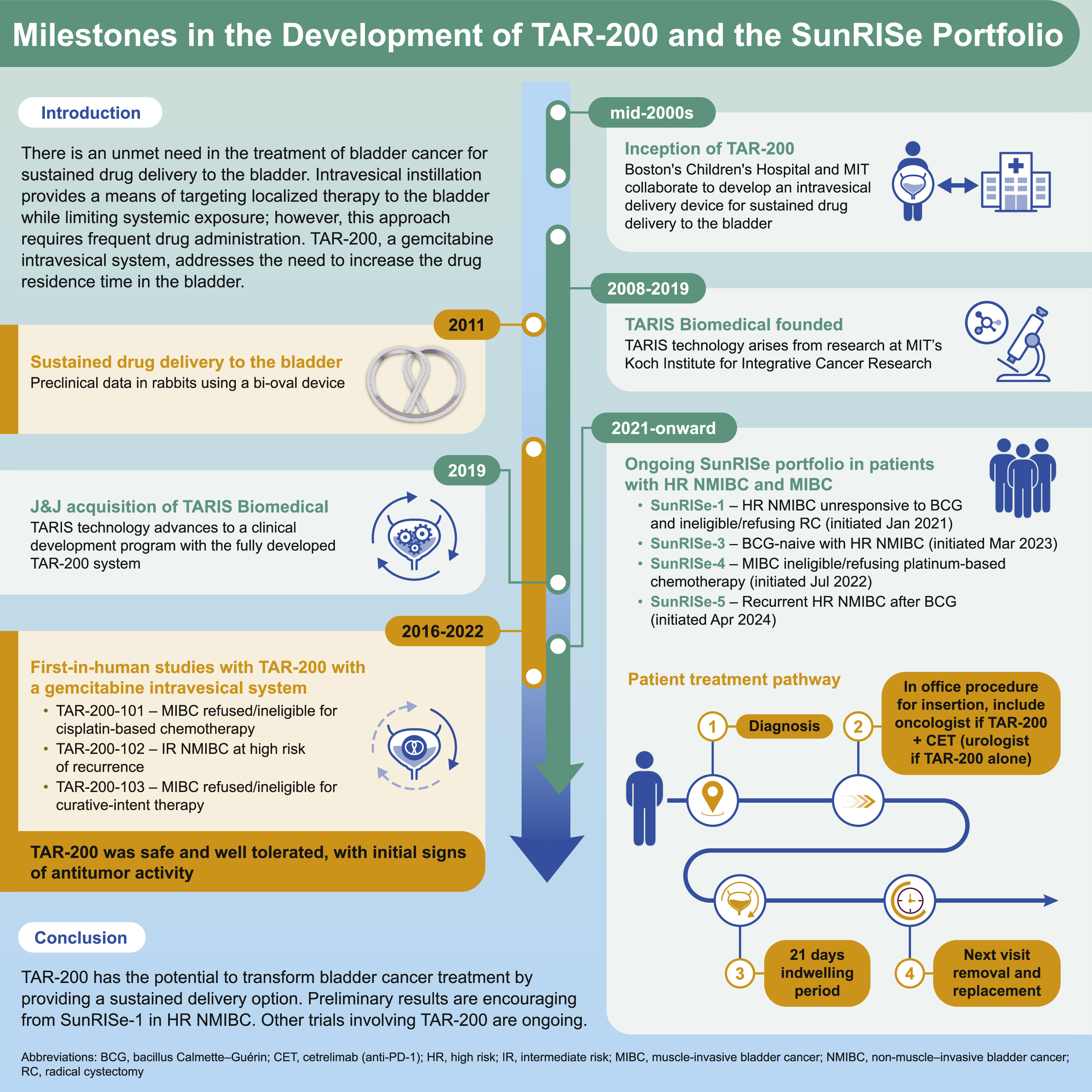
TAR-200 is a single-compartment, targeted releasing system designed to provide sustained intravesical gemcitabine [ ]. TAR-200 elutes a sustained dose of drug to increase the therapeutic window of intravesical exposure and enhance targeted treatment of urothelial carcinoma within the bladder [ ]. The goal is to achieve pharmacologically active target dose levels in the affected tissues, which would not be possible with systemic administration given associated toxicities. There is currently no FDA-approved local continuous drug delivery system for the bladder that addresses these constraints. An intravesical releasing system that facilitates prolonged drug-to-urothelial contact times was hypothesized to improve absorption of the administered drug across the urothelium into deeper layers of the bladder as well as to affect tumor tissue.
2.1
Design features of TAR-200
To address the challenge of delivering sustained local therapy within the bladder lumen to treat bladder cancer, also known as intravesical therapy, the TAR-200 gemcitabine intravesical system is composed of a silicone dual lumen tube with gemcitabine placed within the larger lumen, creating a solid drug core ( Fig. 2 ) [ ].
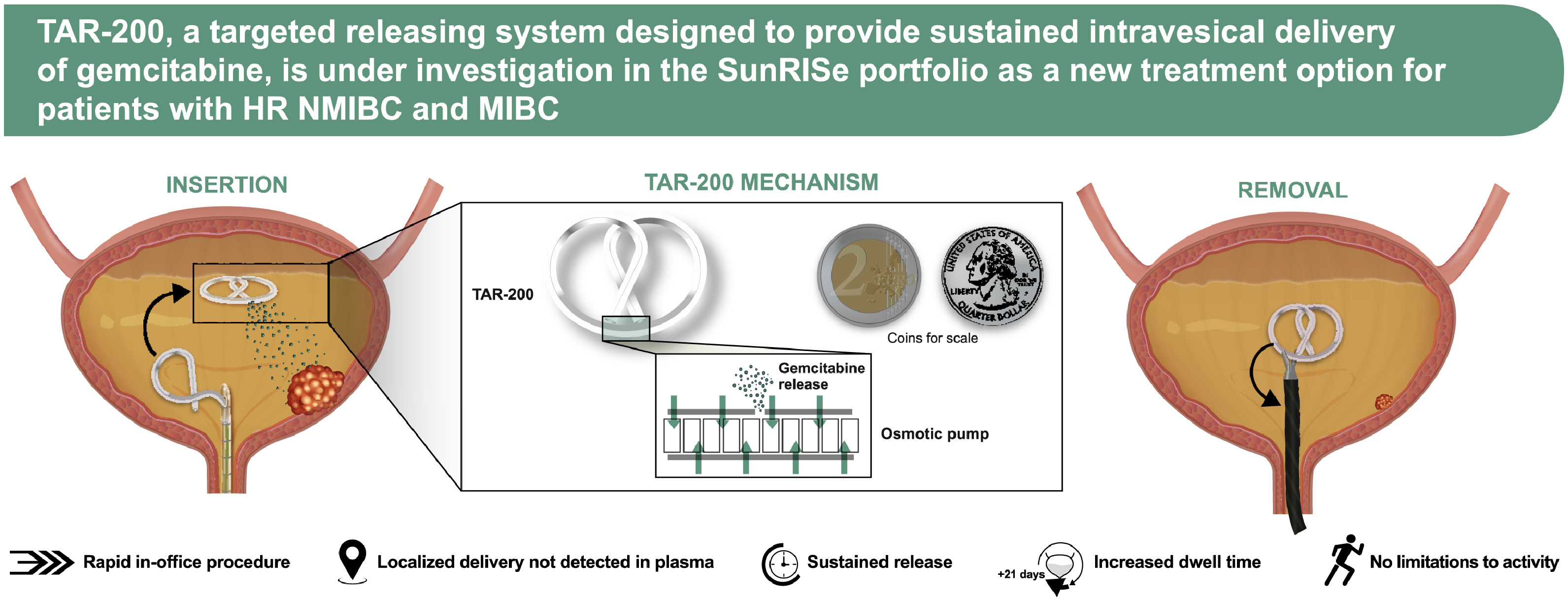
The smaller lumen contains a flexible wire that allows for insertion into the urethra and intravesical coiling to enable successful deployment of the drug delivery system and retention within the bladder during the planned indwelling period [ ]. TAR-200 is uncurled and loaded into a lubricated urinary placement catheter (UPC), which is then inserted into the bladder lumen through the urethra, after which the product is pushed out of the UPC with a stylet. The system returns to its original bi-oval shape after emerging from the UPC. TAR-200 is designed to resist structural collapse and promote retention within the bladder as it is freely mobile and compressible. The size may be compared to a quarter or €2 coin ( Fig. 2 ) [ ]. It is retained within the bladder for an extended period, approximately 3 weeks, and is retrieved via cystoscopy using a grasping forceps [ ].
Intravesical gemcitabine instillations have established efficacy and safety in NMIBC and as standard chemotherapy in combination with cisplatin in the treatment of MIBC [ ]. As a sustained gemcitabine intravesical system, TAR-200 is loaded with one tenth of the usual intravesical gemcitabine dose (225 vs. 2, 000 mg) [ , ], with the goal of optimizing gemcitabine dwell time while minimizing both local and systemic adverse events (AEs) and maximizing efficacy.
Inside the bladder, osmotic pressure regulates gemcitabine release from the TAR-200 internal reservoir, allowing the system to act as an osmotic drug pump ( Fig. 2 ) [ ]. Urea serves as an osmotic agent [ ]. The system is designed to provide localized, sustained gemcitabine delivery into the bladder over an extended period, while minimizing systemic exposure [ ].
2.2
Pharmacokinetics of gemcitabine delivered via TAR-200
TAR-200 is designed to allow for sustained release of gemcitabine over an indwelling period of up to 21 days [ , ]. In animal models, TAR-200 delivered gemcitabine into the urine for at least 7 days at concentrations comparable to target levels ( Fig. 3 A) [ , ]. TAR-200 maintains the gemcitabine therapeutic dose in urine over time, with no gemcitabine detected in the plasma, and the maximum plasma 2′, 2′-difluorodeoxyuridine (dFdU) concentration at any time point is ≤0.35 µg/ml ( Fig. 3 B) [ , ].
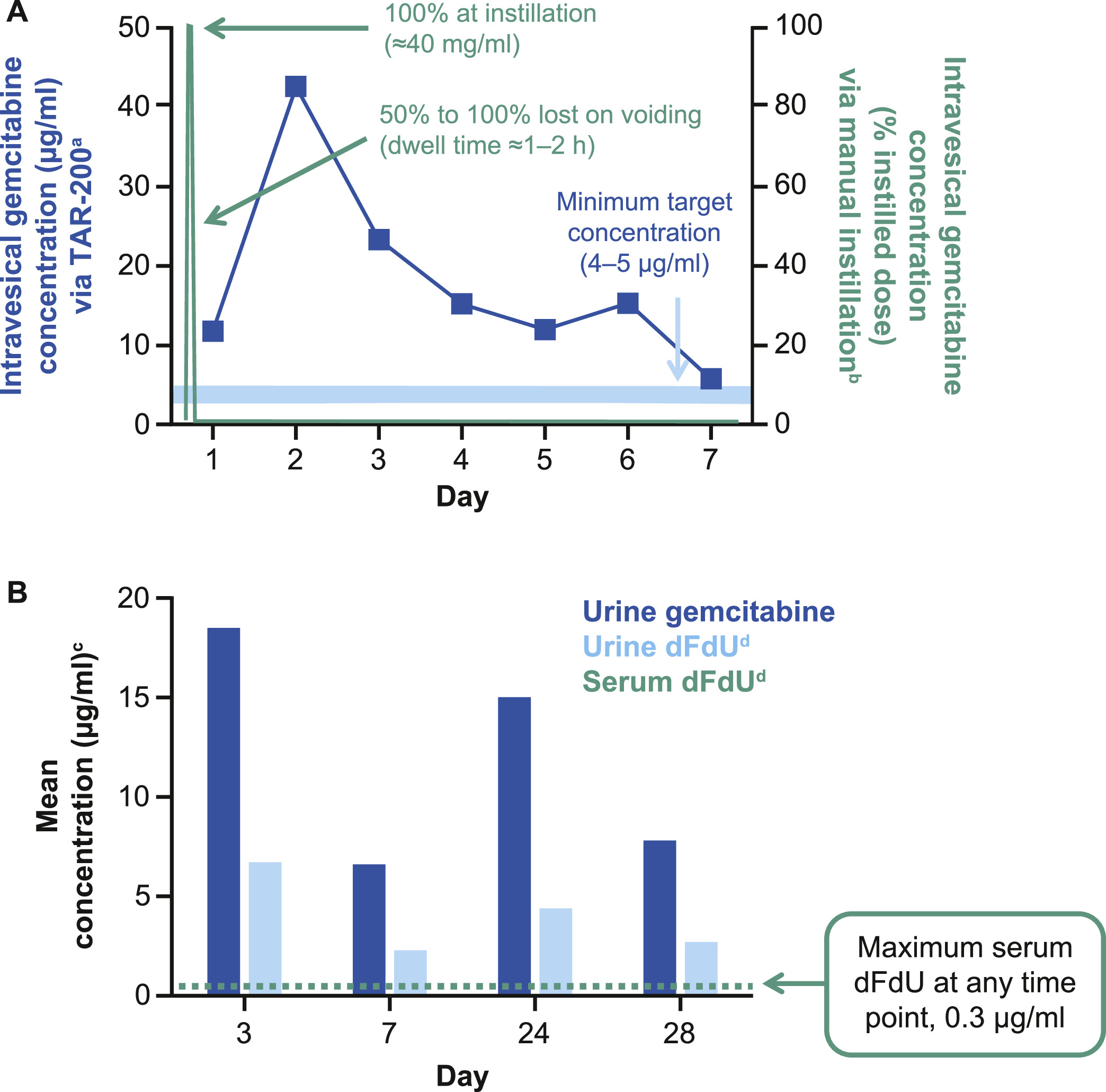
In preclinical models, gemcitabine penetrated deep into bladder tissue following continuous local exposure (unpublished data, TARIS Biomedical). In a rodent model of MIBC, continuous, low-dose intravesical gemcitabine inhibited tumor growth in a concentration-dependent manner [ ]. As gemcitabine is a prodrug, prolonged exposure to a sufficient concentration of gemcitabine may enhance intracellular accumulation of its active tri-phosphorylated metabolite, which impairs DNA synthesis [ ]. In addition, preclinical studies provide evidence of gemcitabine augmentation of antitumor T-cell response [ ].
3
Phase I studies of TAR-200
Three phase I studies (TAR-200-101, TAR-200-102, TAR-200-103) evaluated the safety and initial antitumor activity of TAR-200 monotherapy in intermediate-risk (IR) NMIBC and MIBC [ , , ]. Safety and efficacy results are summarized in Supplementary Table 1 . Across these studies, TAR-200 was well tolerated, with mostly grade 1 to 2 AEs. TAR-200-101 (NCT02722538) evaluated two 7-day cycles of neoadjuvant TAR-200 in patients with MIBC undergoing RC, including those with a residual tumor of >3 cm after TURBT and those who had undergone maximal TURBT (residual tumor <3 cm) [ ]. The most common TAR-200-related AEs were pollakiuria, urinary incontinence, and micturition urgency. Of 20 patients who underwent RC, 10 (50 %) had a pathologic response; 4 (20 %) had a complete response ([CR] T0), and 6 (30 %) had a partial response (<T2, including Ta, T1, and CIS) [ ]. TAR-200-102 (NCT02720367) evaluated two 7-day or two 21-day cycles of TAR-200 in patients with IR NMIBC using a marker lesion/ablation design [ ]. The most common TAR-200-related AEs were urgency, dysuria, and hematuria, essentially all consistent with local urinary tract instrumentation. Interval cystoscopy following TAR-200 placement showed recurrent papillary disease and subsequent complete TURBT; 5 of 12 patients (42 %) had a pathologic CR with pathologically confirmed T0 disease [ ].
TAR-200-103 (NCT03404791) examined 4 consecutive 21-day cycles of TAR-200 in patients with MIBC who were deemed unfit for RC by investigator assessment, or who refused/were ineligible for curative-intent therapy [ ]. The most common TAR-200-related AEs were dysuria, urinary frequency, nocturia, and urethral syndrome. Of 35 patients, 11 had a CR (no evidence of intravesical disease by cystoscopy and biopsy and no evidence of pathologic nodal involvement [>10 mm]), and 3 had a partial response (downstaged intravesical disease [<cT2 and/or decrease in volume on cystoscopy]/no evidence of pathologic nodal involvement [>10 mm]); as such, overall response rate was 40 % [ ].
4
Ongoing phase II and III clinical trials of TAR-200
The TAR-200 clinical development program (now referred to as the SunRISe portfolio) is designed to assess intravesical TAR-200 with or without systemic cetrelimab, a programmed cell death protein 1 (PD-1) inhibitor, as a treatment option for patients with bladder cancer, including patients with HR NMIBC (BCG naive [papillary and CIS] and BCG unresponsive [CIS with and without papillary disease]) and MIBC (neoadjuvant setting and patients ineligible for/refusing RC) ( Fig. 4 , Table ). These studies evaluate TAR-200 in patients who are ineligible for or refuse RC and in the neoadjuvant setting prior to RC. The main eligibility criteria for ongoing SunRISe studies are shown in Supplementary Table 2 .
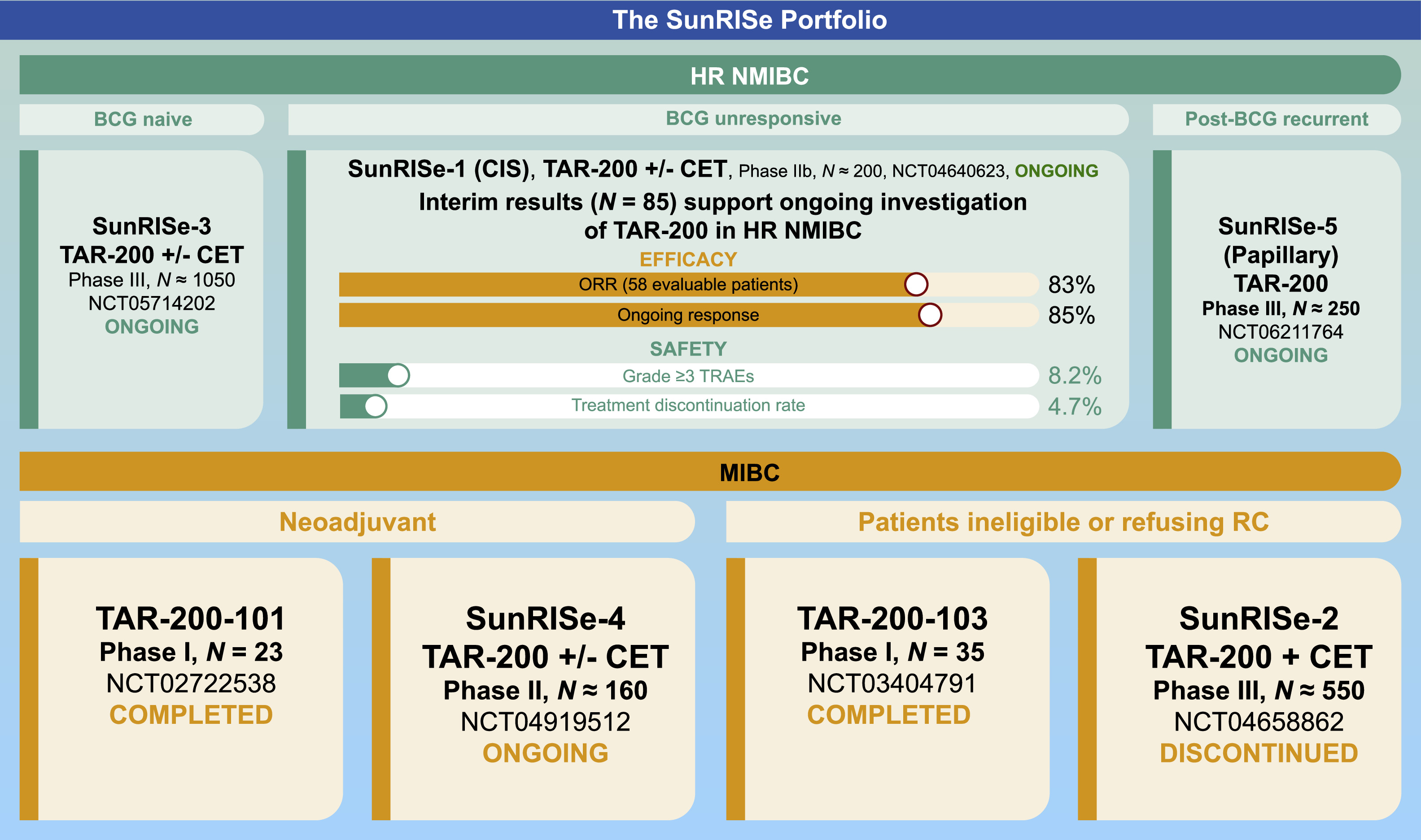
| Study | Phase | Target population | Cohorts/comparator arms | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|
| SunRISe-1 (NCT04640623) [ , ] | IIb | Patients with HR NMIBC unresponsive to BCG/ineligible for or refusing RC ( n ≈ 200) | Cohorts 1-3 (CIS ± papillary disease) Cohort 1: TAR-200 + CET Cohort 2: TAR-200 Cohort 3: CET Cohort 4 (papillary disease only) : TAR-200 | Cohorts 1-3: Overall CR Cohort 4: DFS |
|
| SunRISe-3 (NCT05714202) [ , ] | III | Patients with BCG-naive HR NMIBC ( n ≈ 1050) | Group A: TAR-200 + CET Group B: BCG Group C: TAR-200 | EFS |
|
| SunRISe-4 (NCT04919512) [ , ] | II | Patients with MIBC ineligible for or refusing platinum-based neoadjuvant CT ( n ≈ 160) | Cohort 1: TAR-200 + CET Cohort 2: CET | pCR |
|
| SunRISe-5 (NCT06211764) [ ] | III | Patients with HR NMIBC (recurrent, papillary-only) within first year of BCG and ineligible for or refusing RC ( n ≈ 250) | Experimental: TAR-200 Active comparator: CT (mitomycin C or gemcitabine) | DFS |
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree