Highlights
- •
Testicular cancer makes up 1% of adult tumors and is the most common in young men.
- •
CS II seminoma management options include radiotherapy, chemotherapy, or RPLND.
- •
Chemotherapy and radiotherapy have high cure rates but pose risks of long-term toxicities.
- •
RPLND is gaining interest for low-volume seminoma due to low complications and precise staging.
- •
More research is needed to refine patient selection and the role of adjuvant therapy.
Abstract
Testicular cancer represents 1% of adult neoplasms and is the most common solid malignancy in young men. Of men presenting with seminoma, approximately 20% will have clinical stage (CS) II disease, characterized by enlarged retroperitoneal lymph nodes without further metastasis. A further group of men will present with CS I disease but later experience relapse in the retroperitoneal lymph nodes. The standard treatment for many decades in these patients is either radiotherapy (30–36Gy) or chemotherapy (BEPx3, EPx4). Despite high cure rates with these modalities, concerns persist regarding short and long-term treatment-related toxicities. Survivors of testicular cancer treated with chemotherapy or radiotherapy face increased risks of cardiovascular disease (1.5–6-fold) and secondary malignancies (twice as likely for solid cancers and 5 times for leukemia). An alternative approach explored is primary Retroperitoneal Lymph Node Dissection (RPLND). Several institutional series along with 4 single-arm phase II trials have investigated primary RPLND in men with low-volume retroperitoneal metastases. Herein, we review the evidence, strengths and limitations of the current studies and future for primary RPLND for seminoma.
1
Introduction
Testicular cancer represents 1% of adult neoplasms and is the most common solid malignancy in young men [ , ]. At diagnosis, approximately 90% of cases are germ cell tumors (GCT), categorized as either seminomatous (55%–60%) or nonseminomatous types (40%–45%) [ ]. The majority of seminomas (85%) and nonseminomas (60%) present as clinical stage (CS) I. Patients with testicular cancer can expect to have excellent outcomes, with 5-year survival rates ranging from 99%, 96% and 73% in localized (CS 1), regional (CS II) and distant disease (CS III) [ ].
1.1
Management of CS I seminoma
After local control with radical orchiectomy, there are several management options for CS I seminoma, including active surveillance (AS), adjuvant chemotherapy (carboplatin AU7) and radiotherapy [ , ]. Up to 20% of CS I seminoma patients will experience relapse, usually in the retroperitoneum, and typically within the first 2 to 3 years [ , ]. The use of radiotherapy has generally declined as an adjuvant management strategy for CSI seminoma following the TE19 trial that demonstrated 1 cycle of carboplatin was noninferior to radiotherapy for oncological control (5.3% vs. 4% relapse) and without the risks of long-term secondary GCT [ , ]. Long-term outcomes with carboplatin are infrequently reported, but no increased mortality from circulatory diseases or incidence of secondary nontesticular cancers have been observed [ ]. While no randomized trials have compared all 3 treatment options, a systematic review has shown that disease-free survival (DFS) for CS I seminoma is near 100%, regardless of the treatment modality [ ]. Consequently, AS is often the preferred approach for patients able to comply with follow-up, although relapsed patients may face significantly higher toxicity from cisplatin-based chemotherapy [ , , ].
1.2
Nonsurgical management strategies for CS IIA/IIB seminoma and limitations
Of men presenting with seminoma, approximately 20% will have CS II disease, characterized by enlarged retroperitoneal lymph nodes without further metastasis [ ]. This group is further substratified into CS IIA if the lymph nodes are ≤ 2cm, CS IIB between 2 and 5cm, and CS IIC if greater than 5cm. For many decades, the treatment of patients with clinical stage CSIIA/B seminoma was either radiotherapy or chemotherapy. Radiotherapy delivers 30Gy for CS IIA, and 36Gy with an additional boost to the extended iliac field for CS IIB. Chemotherapy is a combination of bleomycin, etoposide, and cisplatin (BEP) given for 3 cycles, or etoposide and cisplatin (EP) given for 4 cycles [ , ]. While no randomized trials directly compare radiotherapy and chemotherapy, a systematic review demonstrated in CS IIA disease, relapse rates vary between 0 and 5% following radiotherapy and 0% following chemotherapy. For CS IIB patients, relapse rates after radiotherapy ranged from 10 to 21% and 0% to 14% after chemotherapy [ ]. Despite these significant relapse rates, 5-year OS rates for CS IIA/IIB seminoma are above 90% independent of treatment modality [ , ].
Yet, both treatment paradigms carry significant short and long-term treatment-related toxicities [ ]. Radiotherapy, when compared to surveillance or surgery alone, has been shown to double the risk of cardiovascular disease and secondary malignancies (with an incidence of 2%–3%) [ ]. Additionally, patients who receive chemotherapy for GCTs can develop complications including irreversible neurotoxicity (10%), ototoxicity (5%–12%), pulmonary toxicity, which may result in permanent restrictive lung disease (8%) [ ], metabolic syndrome (10%–16%) [ ], and hypogonadism (4%). Additionally, patients are at an approximate 3 to 7-fold increase in cardiovascular mortality [ ] and a 2-fold increase in secondary solid malignancies and leukemia [ , ]. Furthermore, both treatment modalities can harm fertility potential by approximately 20% to 30% [ , ]. When patients experience relapse (affecting 10–20% of cases) and receive multiple lines of treatment, including chemoradiotherapy, the associated complications tend to be synergistic and occur more frequently, resulting in a 4-fold increase in the risk of secondary malignancies (SMN). In men with seminoma, who are diagnosed at a young age and have many decades of expected survival, these long-term complications may negatively impact their quality of life [ ].
Lastly, whilst computed tomography (CT) is commonly used as a primary staging tool for GCTs, it cannot differentiate between benign and malignant lymph nodes. As a result, a size cut-off of 1cm has been traditionally used to trigger treatment decisions. However, this approach may lead to over-treatment with radiation or chemotherapy in 10% to 15% of cases, unnecessarily exposing patients to additional risks.
Therefore, there is a growing push to revise management strategies for young men with seminoma, focusing on minimizing toxicity without compromising survival rates. About 1 strategy explored is reducing the radiation dose (to 27Gy) for CS IIA seminoma, however, it has been associated with higher relapse rates [ ]. Another option is de-escalation therapy (i.e., receiving 2 cycles of EP and 1 cycle of carboplatin AUC7 instead of 4 cycles of EP based on residual metabolic activity identified on FDG-PET). A study by Loriot et al., demonstrated that de-escalation therapy had comparable 3-year progression-free survival (90%) and 2-year overall survival. Further, larger studies and longer follow-up studies are required to confirm these findings [ ].
As such, an alternative strategy which has been explored is primary RPLND, which has now grown of significant interest. Herein, we review the evidence, strengths and limitations of the current studies and future for primary RPLND for Stage II seminoma cancer
2
Primary retroperitoneal lymph node dissection in CS IIA/IIB seminoma
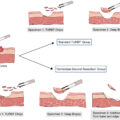
RPLND in early metastatic seminoma was previously displaced by radiotherapy (circa 1980s) due to the high morbidity with surgery. However, advancements in perioperative care and surgical techniques have substantially reduced complication rates. Morbidity now ranges from 5% to 20%, and mortality is less than 0.5% [ , ].
RPLND is an appealing choice for patients with low-volume metastatic seminoma for several reasons: Firstly, seminoma exhibits a more indolent biological behavior and a predictable lymphatic spread pattern compared to nonseminoma diseases, potentially allowing a greater chance of cure with surgery alone. Secondly, RPLND enables precise pathological staging which can avoid overtreatment in those with pathologically negative lymph nodes. It offers an opportunity to reduce the burden of chemotherapy by reserving this treatment only for the small proportion of men who will relapse following RPLND. Finally, RPLND offers an acceptably low risk of short or long-term complications when performed at experienced centers.
Several institutional series along with 4 single-arm phase II trials have investigated primary RPLND as an alternative to radiation or chemotherapy in men with low-volume CS II A/B or relapsed CS I disease ( Table 1 ) [ ]. Across these studies, patients with CS IIA/B experienced 2-year recurrence rates ranging from 10% to 30% (RFS 70%–90%), with survival data still immature due to short-term follow-up [ ]. Variations in surgical techniques/templates, extent of adjuvant chemotherapy use, patient selection criteria, and short follow-up periods complicate direct comparisons with conventional treatment. Nevertheless, there are significant aspects to address and compare.
| SEMS (Surgery in Early Metastatic Seminoma) | COTRIMS (Cologne Trial of Retroperitoneal lymphadenectomy In Metastatic Seminoma) | PRIMETEST (Primary Retroperitoneal Lymph-node Dissection in Patients with Seminomatous Testicular Germ Cell Tumors with Clinical Stage IIA/B) | SWENOTECA (The Swedish Norwegian Testicular Cancer Group Experience) | MSKCC | Indiana | |
|---|---|---|---|---|---|---|
| Prospective | Retrospective | |||||
| Design | Multicenter | Single center | Single center | Multicenter | Single center | Single center |
| Inclusion selection criteria | CS IIA/B, 1–3 cm (1–2 LNs), < x1.5 elevation STM, Synchronous or metachronous within 3y after orchiectomy, No previous adjuvant chemotherapy | CS IIA/B, < 5 cm Negative STM Synchronous or metachronous after surveillance, No previous adjuvant chemotherapy | Unilateral CS IIA/B, < 5 cm HCG < 5 mU/ml Synchronous or metachronous after surveillance or Adjuvant carboplatin for CS I included | CS IIA/IIB, < 3 cm (1–2 LNs) Elevations in HCG permitted Synchronous or metachronous after surveillance Adjuvant carboplatin for CS I included | CS IIA/IIB, < 3 cm < x 1.5 elevation STM, Synchronous or metachronous after surveillance No adjuvant chemotherapy | Isolated RP LNs < 3 cm (1–2 LNs) Elevations in HCG permitted Synchronous or metachronous after surveillance No adjuvant chemotherapy |
| Number of patients | 55 | 30 | 33 | 62 | 45 | 67 |
| Approach | Open | Open or MIS (12%) | Open or MIS (58%) | Open or MIS (65%) | Open | Open |
| RPLND templates | Unilateral and bilateral (35% BL) | Unilateral | Unilateral | Unilateral and bilateral (3%) | Bilateral full template | Unilateral and bilateral (46% BL) |
| Complications (Clavien-Dindo >/= 3) | 2% | 13% | 12% | 5% | 0% | 4.5% |
| Relapsed CSI | 65% | 47% | 73% | 64% | 73% | 72% |
| pN0 | 16% | 10% | 9% | 1.6% | 4% | 1.5% |
| Median FU (months) | 33 | 22 | 32 | 23 | 18 | 22 |
| Adjuvant chemotherapy | 2% | 0% | 0% | 29% | 36% | 3% |
| Relapse rate | 22% | 10% | 30% | 10% | 11% | 17% |
| Infield recurrences | 9% (5/55) | 0% | 9% (3/33) | 6% (4/62) | 0% | 1% (1/67) |
| Contralateral retroperitoneum recurrences | 4% (2/55) | 0% | 12% (4/33) | 0% | 0% | 3% (2/67) |
| Antegrade ejaculation | 95% | 90% | 94% | NR | NR | 98% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree