Radical concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C (MMC) remains the standard of care for squamous cell carcinoma of the anus. Phase III trials over 2 decades have used combined radiation doses of 50 to 60 Gy with concurrent fluoropyrimidines, MMC, or cisplatin in various doses and schedules. Modern radiotherapy techniques allow the production of highly conformal plans, decreasing radiation doses to the organs at risk and ensuring a shorter overall treatment time without the need for treatment breaks. These techniques offer the potential to improve compliance and even escalate doses of radiation.
Key points
- •
Squamous cell carcinoma of the anus is generally a localized disease with a relatively low risk of metastatic disease at presentation; thus, local control is the overriding aim of treatment.
- •
Randomized phase III trials have established the combination of 5-fluorouracil–based chemoradiation concurrent with mitomycin C as the standard of care rather than primary surgery.
- •
The TNM clinical staging system is based on accurate assessment of size (T stage), regional lymph node involvement, and metastatic spread.
- •
Assessment and management of anal cancer are best determined by specialist multidisciplinary teams, and treatment should be carried out in specialized centers.
- •
Future research should attempt to integrate novel biomarker-driven targets, such as anti-CTLA4, anti–programmed cell death, and programmed cell death-ligand 1, into chemoradiation schedules.
Introduction
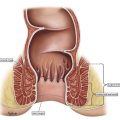
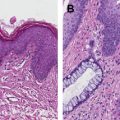
Squamous cell carcinoma of the anus (SCCA) is an uncommon malignancy representing approximately 2% of all gastrointestinal malignancies. The incidence has been increasing over the past decade, probably reflecting more widespread infection with the main causal factor human papillomavirus (HPV).
SCCA is generally a localized disease with a low risk of metastatic disease at presentation. Retrospective studies and randomized trials suggest that locoregional failure is the predominant pattern of relapse, usually in the radiotherapy high-dose volume, and often within the first 2 years following completion of chemoradiation (CRT) treatment. Uncontrolled local recurrence is ultimately responsible for most cancer-related deaths, making local control the primary aim of treatment.
Surgical resection was the standard treatment in the 1970s, which involved removal of the anal canal and a permanent stoma. In the past, radiation alone was also often used to treat SCCA using high doses with split-course schedules and interstitial brachytherapy. The pioneering work of Nigro and colleagues and subsequent confirmatory studies in the United States highlighted the efficacy of CRT using relatively low doses of fractionated radiotherapy (30–45 Gy) combined with 5-fluorouracil (5-FU) and mitomycin C (MMC). Subsequently, 2 randomized trials compared a radiotherapy schedule of 45 Gy boosted with a further 15 to 25 Gy after a gap of 6 weeks against an identical regimen with concurrent 5-FU/MMC. These trials showed radiation alone could result in local control in approximately 45% to 55% of patients. However, both trials confirmed chemoradiotherapy significantly improved outcomes over radiation alone.
Significant toxicity was reported for MMC. So concurrent 5-FU and MMC or 5-FU alone were randomly compared in the CRT component in the RTOG 8704 trial. The addition of MMC significantly improved both disease-free survival (DFS) and colostomy-free survival (CFS). Thus, a series of randomized trials all confirmed the efficacy of concurrent CRT with 5-FU/MMC and relegated the role of surgery to salvage of CRT failures. The small Action Clinique Coordonees en Cancerologie Digestive (ACCORD-03) trial, in contrast, used concurrent 5-FU/cisplatin.
The standard of care both in Europe and North America is 5-FU/MMC CRT and is recommended in guidelines. This schedule results in complete tumor regression in 80% to 90%, with a high level of permanent disease control particularly for cT1/T2 tumors. The 5-year overall survival (OS) reached 78% in the MMC arm of the RTOG 9811 trial, 71% in the CRT-alone arms without neoadjuvant chemotherapy (NACT) in ACCORD-03, and 79% in the MMC arm of Anal Cancer Trial II (ACT II).
Preservation of sphincter function is usually achieved; but with doses of 50 to 60 Gy, there is a risk of fecal incontinence. In more advanced T3/T4 cancers with nodal metastases, it is more difficult to achieve local control ; a substantial proportion of such patients will fail within 2 years. In the ACT II, patients with cT3/T4 cancers and nodal metastases had a 3-year progression-free survival (PFS) of 63%.
Randomized phase III trials by RTOG 9811, the ACCORD-03 phase III trial, and the ACT II trial failed to show any additional benefit in terms of PFS/DFS by increasing the radiotherapy boost dose or replacing MMC with cisplatin during CRT. Additional cisplatin-based chemotherapy given as induction before CRT or as maintenance or consolidation after CRT has not improved outcomes ( Table 1 ).
| Trial Name (Years) | Number of Patients | Design | RT Dose | Comparison 1 | Comparison 2 | Primary End Point |
|---|---|---|---|---|---|---|
| RTOG 9811 (1998–2005) (Ajani et al, 2008) | 644 | NACT cisplatin/5-FU Then 5-FU/cisplatin/RT (ie, 4 courses) vs 5-FU/MMC/RT | Phase I 45 Gy/25# in 5.0–6.5 wk T3/T4, N+, or residual T2 boost to 54–59 Gy | NACT with 5-FU 1000 mg/m 2 days 1–4, 29–32, and cisplatin 75 mg/m 2 then CRT 5-FU/cisplatin vs MMC 10 mg/m 2 days 1 and 29 and 5-FU 1000 mg/m 2 days 1–4, 29–32 CRT | DFS | |
| ACCORD-03 (1999–2005) (Peiffert et al, 2012) | 307 | 2 × 2 factorial NACT (5-FU/cisplatin, 2 cycles) vs no NACT Standard vs high-dose boost for responders | Phase I 45 Gy/25#/33 d 3-wk gap then 15-Gy boost standard arms; 20 Gy–25 Gy boost (high-dose arms) for responders; 40% received brachytherapy boost | NACT with 5-FU 800 mg/m 2 days 1–4, 29–32 and cisplatin 80 g/m 2 on days 1 and 29; then CRT 5-FU/cisplatin on days as mentioned earlier with RT | Dose escalation of RT boost 15-Gy boost standard arms 20-Gy–25-Gy boost (high-dose arms) for responders | CFS Secondary end points: local control, OS, and cancer-specific survival |
| ACT II (UKCCCR) (2001–2008) (James et al, 2013) | 940 | 2 × 2 factorial 5-FU/MMC vs 5-FU cisplatin CRT and consolidation 5-FU/cisplatin vs control | Phase I 30/6 Gy/17# in 3.5 wk then phase II 19.8 Gy/11# conformal Total 50.4 Gy/28#/38 d no gap | Cisplatin 60 mg/m 2 d 1 and 29 vs 12 mg/m 2 MMC day 1 with 5-FU 1000 mg/m 2 days 1–4, 29–32 CRT | Consolidation 2 courses 5-FU 1000 mg/m 2 and cisplatin 60 mg/m 2 | Relapse-free survival |
SCCA regresses slowly following radiation or CRT. In early trials a 6 to 8 week planned gap between the completion of CRT and a radiotherapy boost allowed the acute toxicity of skin and mucosal surfaces to resolve. During this interval the tumor would shrink, and permit an interstitial implant to the smallest possible volume – minimizing the risk of radiation induced necrosis. This strategy also allowed selection of nonresponders for salvage either by dose-escalation of the radiotherapy boost or by surgical resection. Later trials continued this approach although shortened the interval. However this practice defied radiobiological principles, because the gap allows the cancer cells to repopulate toward the end of radiotherapy, which in theory partly accounts for treatment failure. The only trial that obeyed these constraints and mandated a continuous schedule and prohibited a planned interval was the ACT II trial.
In this article, the authors discuss the existing evidence basis for the current treatment regimens. The authors review target delineation, optimized radiotherapy techniques, dose-escalation with external-beam radiotherapy (EBRT) or brachytherapy, and chemotherapy. The authors also speculate on novel approaches in terms of the integration of biological agents and immunotherapy, which are being developed for SCCA.
Introduction
Squamous cell carcinoma of the anus (SCCA) is an uncommon malignancy representing approximately 2% of all gastrointestinal malignancies. The incidence has been increasing over the past decade, probably reflecting more widespread infection with the main causal factor human papillomavirus (HPV).
SCCA is generally a localized disease with a low risk of metastatic disease at presentation. Retrospective studies and randomized trials suggest that locoregional failure is the predominant pattern of relapse, usually in the radiotherapy high-dose volume, and often within the first 2 years following completion of chemoradiation (CRT) treatment. Uncontrolled local recurrence is ultimately responsible for most cancer-related deaths, making local control the primary aim of treatment.
Surgical resection was the standard treatment in the 1970s, which involved removal of the anal canal and a permanent stoma. In the past, radiation alone was also often used to treat SCCA using high doses with split-course schedules and interstitial brachytherapy. The pioneering work of Nigro and colleagues and subsequent confirmatory studies in the United States highlighted the efficacy of CRT using relatively low doses of fractionated radiotherapy (30–45 Gy) combined with 5-fluorouracil (5-FU) and mitomycin C (MMC). Subsequently, 2 randomized trials compared a radiotherapy schedule of 45 Gy boosted with a further 15 to 25 Gy after a gap of 6 weeks against an identical regimen with concurrent 5-FU/MMC. These trials showed radiation alone could result in local control in approximately 45% to 55% of patients. However, both trials confirmed chemoradiotherapy significantly improved outcomes over radiation alone.
Significant toxicity was reported for MMC. So concurrent 5-FU and MMC or 5-FU alone were randomly compared in the CRT component in the RTOG 8704 trial. The addition of MMC significantly improved both disease-free survival (DFS) and colostomy-free survival (CFS). Thus, a series of randomized trials all confirmed the efficacy of concurrent CRT with 5-FU/MMC and relegated the role of surgery to salvage of CRT failures. The small Action Clinique Coordonees en Cancerologie Digestive (ACCORD-03) trial, in contrast, used concurrent 5-FU/cisplatin.
The standard of care both in Europe and North America is 5-FU/MMC CRT and is recommended in guidelines. This schedule results in complete tumor regression in 80% to 90%, with a high level of permanent disease control particularly for cT1/T2 tumors. The 5-year overall survival (OS) reached 78% in the MMC arm of the RTOG 9811 trial, 71% in the CRT-alone arms without neoadjuvant chemotherapy (NACT) in ACCORD-03, and 79% in the MMC arm of Anal Cancer Trial II (ACT II).
Preservation of sphincter function is usually achieved; but with doses of 50 to 60 Gy, there is a risk of fecal incontinence. In more advanced T3/T4 cancers with nodal metastases, it is more difficult to achieve local control ; a substantial proportion of such patients will fail within 2 years. In the ACT II, patients with cT3/T4 cancers and nodal metastases had a 3-year progression-free survival (PFS) of 63%.
Randomized phase III trials by RTOG 9811, the ACCORD-03 phase III trial, and the ACT II trial failed to show any additional benefit in terms of PFS/DFS by increasing the radiotherapy boost dose or replacing MMC with cisplatin during CRT. Additional cisplatin-based chemotherapy given as induction before CRT or as maintenance or consolidation after CRT has not improved outcomes ( Table 1 ).
| Trial Name (Years) | Number of Patients | Design | RT Dose | Comparison 1 | Comparison 2 | Primary End Point |
|---|---|---|---|---|---|---|
| RTOG 9811 (1998–2005) (Ajani et al, 2008) | 644 | NACT cisplatin/5-FU Then 5-FU/cisplatin/RT (ie, 4 courses) vs 5-FU/MMC/RT | Phase I 45 Gy/25# in 5.0–6.5 wk T3/T4, N+, or residual T2 boost to 54–59 Gy | NACT with 5-FU 1000 mg/m 2 days 1–4, 29–32, and cisplatin 75 mg/m 2 then CRT 5-FU/cisplatin vs MMC 10 mg/m 2 days 1 and 29 and 5-FU 1000 mg/m 2 days 1–4, 29–32 CRT | DFS | |
| ACCORD-03 (1999–2005) (Peiffert et al, 2012) | 307 | 2 × 2 factorial NACT (5-FU/cisplatin, 2 cycles) vs no NACT Standard vs high-dose boost for responders | Phase I 45 Gy/25#/33 d 3-wk gap then 15-Gy boost standard arms; 20 Gy–25 Gy boost (high-dose arms) for responders; 40% received brachytherapy boost | NACT with 5-FU 800 mg/m 2 days 1–4, 29–32 and cisplatin 80 g/m 2 on days 1 and 29; then CRT 5-FU/cisplatin on days as mentioned earlier with RT | Dose escalation of RT boost 15-Gy boost standard arms 20-Gy–25-Gy boost (high-dose arms) for responders | CFS Secondary end points: local control, OS, and cancer-specific survival |
| ACT II (UKCCCR) (2001–2008) (James et al, 2013) | 940 | 2 × 2 factorial 5-FU/MMC vs 5-FU cisplatin CRT and consolidation 5-FU/cisplatin vs control | Phase I 30/6 Gy/17# in 3.5 wk then phase II 19.8 Gy/11# conformal Total 50.4 Gy/28#/38 d no gap | Cisplatin 60 mg/m 2 d 1 and 29 vs 12 mg/m 2 MMC day 1 with 5-FU 1000 mg/m 2 days 1–4, 29–32 CRT | Consolidation 2 courses 5-FU 1000 mg/m 2 and cisplatin 60 mg/m 2 | Relapse-free survival |
SCCA regresses slowly following radiation or CRT. In early trials a 6 to 8 week planned gap between the completion of CRT and a radiotherapy boost allowed the acute toxicity of skin and mucosal surfaces to resolve. During this interval the tumor would shrink, and permit an interstitial implant to the smallest possible volume – minimizing the risk of radiation induced necrosis. This strategy also allowed selection of nonresponders for salvage either by dose-escalation of the radiotherapy boost or by surgical resection. Later trials continued this approach although shortened the interval. However this practice defied radiobiological principles, because the gap allows the cancer cells to repopulate toward the end of radiotherapy, which in theory partly accounts for treatment failure. The only trial that obeyed these constraints and mandated a continuous schedule and prohibited a planned interval was the ACT II trial.
In this article, the authors discuss the existing evidence basis for the current treatment regimens. The authors review target delineation, optimized radiotherapy techniques, dose-escalation with external-beam radiotherapy (EBRT) or brachytherapy, and chemotherapy. The authors also speculate on novel approaches in terms of the integration of biological agents and immunotherapy, which are being developed for SCCA.
Strategies for treating localized anal canal cancer
Radical Surgery
Up until the mid1980s, the standard treatment of SCCA involved surgery with a permanent colostomy. Local failure rates were reported between 27% and 47% and 5-year OS rates between 40% and 70% (depending on stage and extent of the disease at presentation). Although there has never been a randomized trial comparing radical surgery with radiotherapy or CRT, CRT achieves good local control without the inevitability of a permanent stoma.
Neoadjuvant/Induction Chemotherapy
NACT is widely used in cancers of the head and neck (SCC of the head and neck) before surgery or definitive CRT. In Europe a small pilot study treated SCCA with neoadjuvant 5-FU and cisplatin and showed an 82% response rate, with 27% achieving a complete clinical response (CCR). A further study integrated neoadjuvant 5-FU/cisplatin and showed CCR and partial response (PR) rates of 10% and 51% after NACT, 67% and 28% after CRT, and 93% and 5%, respectively, after all treatment was completed.
In the United States, the Cancer and Leukemia Group B (CALGB 9281) phase II study, for patients with larger, more advanced SCCA (cT3/T4) or with lymph node involvement (cN2/N3), evaluated induction chemotherapy using 5-FU (1000 mg/m 2 days 1–4 and 29–32) and cisplatin (100 mg/m 2 on days 1 and 29) before CRT. The results showed 8 patients (18%) with CCR and 21 with PR in the 44 evaluable patients, and only a single patient had disease progression. After 4 years of follow-up, 27 of 44 (61%) were disease free.
Two randomized studies (RTOG 9811 and ACCORD-03) examined NACT based on the success of these results. Both incorporated split-course CRT. Additionally RTOG 9811 used 5-FU/cisplatin as the radio-sensitization for the CRT part of the treatment in place of MMC. The results were virtually identical for the CALGB 9281 trial and the RTOG 9811 trial; but in the RTOG 9811 trial, only 26% of patients had tumors greater than 5 cm and positive nodes. Neither of the randomized trials documented the clinical response rates to NACT and whether responding patients had better locoregional control, disease-free survival, and OS.
It remains unclear from the reports of the RTOG 9811 trial whether total dose and/or planning target volumes were modified if patients achieved CCR or near CCR with NACT. There are no specific instructions in protocol. Hence, this may have introduced bias in the different arms, as the authors do not know whether the total dose of radiotherapy differed between induction and the MMC control arm. The studies also fail to report whether the gross target volume and clinical target volume were substantially different between arms or whether the pattern of relapse in the induction arm was different to the MMC control arm.
Radiotherapy
External Beam Radiation Alone
EBRT was originally used as a single modality without chemotherapy. A control rate at the primary site of 75% and OS rates at 5 years of 61% were reported. In a series of 270 patients, local control was 90% for tumors less than 4 cm at 10 years but only 65% at 10 years for tumors greater than 4 cm. An early pooled analysis did not show a benefit for 5-FU/MMC in T1 tumors. Some units, therefore, continue to treat small T1 tumors with radiation alone, although the ACT I trial suggested CRT is more effective, even for T1 tumors, with a hazard ratio in favor of CRT similar to T2 and T3 cancers.
Retrospective studies suggest most recurrences occur locally at the primary site often with involvement of adjacent structures and within the high-dose area. Others have reported higher rates of regional recurrence when lower elective nodal doses are used, that is, inguinal failure (40%), common iliac (20%), external iliac (10%), internal iliac (10%), and presacral region (15%). Nigro reported that tumors greater than 4 cm in size were more difficult to control. Patients with lesions less than 3 cm in diameter and those treated strictly according to the protocol did significantly better than those with larger lesions and those whose treatment did not comply with the protocol.
The optimal radiotherapy dose and fractionation for each clinical stage remains unknown. The data from the trials are muddied by limited information on the actual doses received, the overall treatment time (OTT), and the pattern of failure (local, locoregional, or distant). There are no data capturing the sites of locoregional failure in terms of the field size and target delineation, that is, within, marginal to, or outside of the prescribed dose for that radiotherapy field.
Preliminary data from the ACT II trial also suggest failure is usually in-field and that locoregional failure in the elective nodal volume treated with 30.6 Gy was unusual, suggesting insufficient radiation dose or intrinsic radioresistance rather than inadequate clinical target volumes. Recently, a prospective phase II study evaluating intensity-modulated radiotherapy (IMRT) (RTOG O529) suggested a reduction in acute toxicity compared with the RTOG 9811 trial using 2-dimensional and 3-dimensional techniques. IMRT may reduce the need for treatment breaks, which impact adversely on local control and allow dose-escalation in patients at high risk of local recurrence (T4N0 and T3-4N+). A national IMRT protocol for anal cancer has been developed in the UK ( http://www.analimrtguidance.co.uk/analimrtguidance.pdf ).
Radiotherapy dose
In general, larger, more advanced cancers are more difficult to control than smaller ones and require higher doses of radiation, which in turn causes more morbidity. Identification of the optimal radiotherapy dose either with RT alone or CRT has been a challenge. Nigro originally used a low dose of radiation, 30 Gy, in the CRT schedule as a preoperative neoadjuvant treatment before surgical resection. RTOG 8314 delivered 40.08 Gy in 4.5 to 5.0 weeks to the pelvis and perineum. Other prospective series increased the dose to 40 to 45 Gy delivered in 1.8 to 2.0 Gy per fraction and achieved a complete response rate of 86%. Studies in older frail adults have also used lower doses for small tumors.
Traditionally, a boost to the tumor with an interval after 45 Gy EBRT with or without chemotherapy has been used to achieve local control in high-risk patients. Most early trials used this schedule. The ACCORD-03 trial, randomized eligible patients between standard (15 Gy) and higher boost doses (20–25 Gy), but also allowed the use of a higher radiotherapy boost dose if a poor clinical response was observed. The protocol allowed a 3-week gap between CRT and the boost, which will have diluted the effect of the higher dose. In addition, some trials introduced bias by allowing or even encouraging the investigator to boost with higher total doses if a lack of response in the primary was observed. The RTOG 9811 trial seems to recommend a minimum total dose of 45 Gy for good responders in T1/T2 cancers and higher doses for more advanced tumors or poor responders. The ACT II trial is the only trial that mandated a standard dose of 50.4 Gy to the primary tumor for all stages over 28 daily fractions using a 2-phase technique irrespective of response, with no gap between the phases.
RTOG 9208 assessed an escalation of the radiotherapy dose to 59.6 Gy over 8.5 weeks with a mandatory 2-week gap. This schedule did not seem to improve local control but showed an increased 1- to 2-year colostomy rate compared with the RTOG 8704 outcomes whereby patients received a dose of only 45.0 to 50.4 Gy in 1.8 Gy per fraction. A further 20 patient cohort was recruited with the same dose without treatment breaks, also showed no statistical significant difference in local relapse rate.
Higher radiation doses using IMRT are proposed to improve these results, although there are concerns that the function of the sphincter mechanism will be compromised with greater than 54 to 56 Gy leading to fecal incontinence, poor function, and the eventual need for a defunctioning colostomy. Long-term fecal incontinence in prostate cancer seems to correlate with the mean dose to the anal-sphincter region, with a cutoff when doses of greater than 40 Gy confer more risk. Recent studies suggest that poor sphincter control correlates with doses of 53 Gy or greater received by the lateral extent of the anal sphincter region.
Late complications of treatment represented 14.5% of the reasons for having a colostomy in the ACCORD-03 trial, that is, 9 of 307 (3%) of the overall population. The RTOG 9811 trial reported severe long-term toxic effects in both arms of 11% and 10%, and 5% of patients required a colostomy for late effects. In ACT II, 14 of 112 (12.5%) patients requiring a posttreatment colostomy needed this because of morbidity, that is, 14 of 940 (1.5%) overall.
In general, adverse late effects reflect the total radiation dose rather than the type or dose of chemotherapy. With the exception of ACT II, total radiation doses have not been clearly documented in reports of the randomized trials. Sadly ACT II has not reported late toxicity, so a dose response model is, therefore, difficult to calculate.
Elective dose
The appropriate dose to elective nodal areas is also controversial. RTOG 8704 and RTOG 9811 prescribed doses of 30.6 Gy, 36 Gy, and 45 Gy to different areas of clinically negative nodes depending on their anatomic location. However, both the initial United Kingdom Coordinating Committee on Cancer Research (UKCCCR) and European Organization for Research and Treatment of Cancer (EORTC) trials used doses of 45 Gy to elective nodal stations. The RTOG 0529 prescribed 42 to 45 Gy to elective areas that appeared uninvolved on imaging and 50.4 Gy to inguino-pelvic lymph nodes less than 3 cm in size. Lymph nodes larger than 3 cm in size were treated to the same dose as the primary tumor, that is, 54 Gy. The elective nodal dose in ACT II was only 30.6 Gy in 17 daily fractions with large parallel, opposed fields. Others have documented that CRT to elective nodal volumes (inguinal area and whole pelvis) over 4 weeks using a dose of 36 Gy achieved excellent nodal control. Hence, a total of 30 Gy may be sufficient to treat microscopic nodal deposits and small-volume (<2 cm) macroscopic disease.
Brachytherapy
Brachytherapy is a highly conformal treatment that can deliver a high dose to the primary tumor, sparing surrounding normal tissues and the contralateral mucosa and sphincter. Implantation requires local or general anesthesia and demands skill and experience from the operator. Brachytherapy as a single modality is not recommended but may prove useful as a high-dose boost following response to CRT. Often a dose of 45 to 50 Gy using CRT is followed by a 15- to 20-Gy boost, delivered using interstitial brachytherapy. To avoid the risk of necrosis and/or treatment failure, the technique is probably best restricted to tumors that extend less than 50% of the circumference of the anal canal, are less than 5 to 10 mm in thickness, and are less than 5 cm in length. Brachytherapy is a potentially useful technique both for the definitive and recurrent/salvage settings, but there is no consensus on the optimal fractionation schedule. Double-plane or volume implants may be necessary, depending on the extent of the tumor. There is a risk of late necrosis and ongoing radiation proctitis. Recent reports of multiparametric MRI/computed tomography (CT) image adapted brachytherapy show the technique allows excellent dose distribution, but the expertise is limited.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree