Esophagogastric cancer is a worldwide health problem. The addition of the epidermal growth factor receptor 2 (HER2)-directed antibody trastuzumab to chemotherapy increased the overall survival of patients with metastatic HER2-positive esophagogastric cancer. This article discusses the available data to support HER2 as validated biomarker and recently completed and ongoing clinical trials of HER2-directed agents in metastatic and localized disease. Also reviewed is the mechanisms of resistance for HER2-directed therapy and ongoing research strategies including new imaging techniques and studies with patient-derived xenografts.
Key points
- •
Approximately 20% of EGC have ERBB2 (HER2) gene amplification or oncoprotein overexpression and HER2-directed therapy improves the outcome in metastatic disease.
- •
Immunohistochemistry analysis for EGC has different parameters than for breast cancer and requires a trained pathologist for evaluation.
- •
Trastuzumab is the standard treatment in combination with chemotherapy for HER2-positive advanced EGC.
- •
Studies investigating the use of HER2-directed therapy in early stages of disease are ongoing.
- •
HER2-directed imaging and patient-derived xenografts might be a useful tool to guide patient treatment and research.
Introduction
Gastric cancer is the third leading cause of cancer-related deaths worldwide. Most patients are diagnosed with advanced disease and have a median overall survival (OS) of less than 1 year when treated with available cytotoxic chemotherapy. HER2 is a validated therapeutic target in metastatic esophagogastric cancer (EGC). The HER2 proto-oncogene is located on chromosome 17q21 and encodes the 185-kDa transmembrane tyrosine kinase receptor HER2 (also known as HER2/neu, ERBB2, p185). Similarly to breast cancer, the prognosis of patients with HER2 amplified EGC improved when anti-HER2 therapy was added to conventional chemotherapy. Overall, the HER2-positivity rate is around 20% for EGC, with variances according to tumor histologic subtype and location. The rates of HER2 positivity are the highest in esophagogastric junction and stomach cardia tumors, which is up to 30%. In the mid and distal stomach, it is approximately 15% to 20%, and less than 5% of diffuse or signet ring cell type tumors are positive for HER2 amplification.
Introduction
Gastric cancer is the third leading cause of cancer-related deaths worldwide. Most patients are diagnosed with advanced disease and have a median overall survival (OS) of less than 1 year when treated with available cytotoxic chemotherapy. HER2 is a validated therapeutic target in metastatic esophagogastric cancer (EGC). The HER2 proto-oncogene is located on chromosome 17q21 and encodes the 185-kDa transmembrane tyrosine kinase receptor HER2 (also known as HER2/neu, ERBB2, p185). Similarly to breast cancer, the prognosis of patients with HER2 amplified EGC improved when anti-HER2 therapy was added to conventional chemotherapy. Overall, the HER2-positivity rate is around 20% for EGC, with variances according to tumor histologic subtype and location. The rates of HER2 positivity are the highest in esophagogastric junction and stomach cardia tumors, which is up to 30%. In the mid and distal stomach, it is approximately 15% to 20%, and less than 5% of diffuse or signet ring cell type tumors are positive for HER2 amplification.
Diagnosis
Based on the benefit seen from HER2-directed therapy for advanced EGC, testing is currently recommended to all patients on diagnosis. Accurate assessment of HER2 status is essential to determine which patients will benefit from therapy. The test for HER2 in breast cancer is performed using immunohistochemistry (IHC), which shows the HER2 protein expression, and/or fluorescence in situ hybridization (FISH) or chromogenic in situ hybridization, which detects gene amplification.
For EGC, IHC evaluation uses parameters distinct from breast cancer. It is suggested that application of breast cancer scoring to gastric cancer may produce up to 50% false-negative rates on IHC. In comparison with breast cancer, EGC has a higher incidence of tumor heterogeneity. Pathologists should be aware that more focal staining is common in EGC. Expression is mainly restricted to intestinal-type gland-forming cells, and there is incomplete, often basolateral or only lateral membranous IHC staining distribution, appearing as discontinuous HER2 membrane reactivity. A positive IHC in the gastric cancer–specific scoring includes a strong but incomplete membrane staining in greater than or equal to 10% of the cells or greater than or equal to five clustered cells. These criteria showed a high level of concordance between the IHC and FISH testing.
The current recommendation for HER2 evaluation in EGC is that IHC should be the first test performed, using validated assays. Results of IHC 3+, or a FISH ratio of the average HER2 gene copy number to chromosome 17 centromere (HER2/CEP17) greater than or equal to 2.0, are considered positive. Samples with equivocal IHC scores of 2+ should be retested by FISH or other in situ methods. IHC 0 to 1+ is considered HER2 negative.
Locally advanced disease
Currently, there are no definitive data supporting the use of anti-HER2 agents in the adjuvant or neoadjuvant treatment of patients with EGC ( Table 1 ). Based on the encouraging results published with anti-HER2 therapies for localized breast cancer and together with the findings in advanced esophagogastric tumors, anti-HER2 therapies are now under investigation for earlier disease stages.
| Study | Phase | Treatment Line | N | Treatment | Response Rate (%) | Overall Survival (mo) | P |
|---|---|---|---|---|---|---|---|
| TOGA | III | 1 | 594 | Cis + 5-FU | 35 | 11.1 | P = ·0046 |
| Cis + 5-FU + Trastuzumab | 47 | 13.8 | |||||
| LOGIC | III | 1 | 545 | XELOX | 39 | 10.5 | P = .3492 |
| XELOX + Lapatinib | 53 | 12.2 | |||||
| TyTAN | III | ≥2 | 261 | Paclitaxel | 9 | 8.9 | P = .1044 |
| Paclitaxel + Lapatinib | 27 | 11.0 | |||||
| GATSBY | II/III | ≥2 | 415 | Taxane | 19.6 | 8.6 | P = .86 |
| TDM-1 | 20.6 | 7.9 | |||||
| NEOHX | II | Perioperative | 63 | XELOX-T | 39 | NR | — |
| AIO-STO-0310 | II | Perioperative | 57 | FLOT-T | 56.2 | NR | — |
In 2013, a Spanish, multicenter, phase II study called NEOHX was initially presented and the final results were updated in 2015 (NCT01130337). This study evaluated the efficacy and toxicity profile for perioperative XELOX-T (capecitabine, oxaliplatin, and trastuzumab) followed by 12 cycles of adjuvant trastuzumab in monotherapy for patients with HER2-positive locally advanced but resectable stomach or esophagogastric-junction adenocarcinoma. The primary end point was disease-free survival (DFS) at 18 months and secondary end points included pathologic complete response rate (pCR), R0 resection rate, overall response rate, toxicity of preoperative treatment, and biomarker expression. A total of 63 patients were included. Before surgery, five patients stopped treatment because of toxicity. The overall response rate was 39%. Surgery was performed in 31 patients and 28 (78%) had an R0 procedure. Three patients had pCR (8.3%). After surgical resection, postoperative XELOX-T was administered to 24 patients, 22 of whom underwent trastuzumab monotherapy. With a median follow-up of 24.1 months, the 18-month DFS was 71% (95% confidence interval [CI], 53%–83%), the 24-month DFS was 60%, and median DFS and OS had not been reached.
The AIO-STO-0310, a German study reported in 2014, was a multicenter phase-II trial testing perioperative chemotherapy with 5-FU, leucovorin, docetaxel, and oxaliplatin (FLOT) in combination with trastuzumab for patients with HER2-positive, locally advanced, resectable adenocarcinoma of the gastroesophageal junction or stomach (NCT01472029). The primary end point was the rate of centrally tested pCR. Fifty-seven patients were included and 69% of them were able to complete all treatment proposed in the study. Twelve patients (21.1%) had a pCR. The R0 resection rate was 93%. There were no unexpected treatment safety findings as described by the investigators.
Among the ongoing trials there is a European study (INNOVATION-TRIAL) and the Radiation Therapy Oncology Group (RTOG) 1010. The first is a phase II study of perioperative treatment of patients with HER2-positive resectable gastric or gastroesophageal junction adenocarcinoma (NCT02205047). Patients are randomized into one of the three arms: (1) standard chemotherapy (cisplatin/capecitabine or cisplatin/5-fluorouracil), (2) standard chemotherapy plus trastuzumab, or (3) standard chemotherapy plus trastuzumab and pertuzumab. Pertuzumab is a humanized monoclonal antibody that binds to extracellular dimerization domain II of HER2, and inhibits heterodimerization of HER2 with other HER family members receptors, especially HER2–HER3, which is the most potent signaling HER heterodimer. The primary end point is the rate of major pathologic response (ie, <10% viable tumor cells present in the pathologic specimen).
The RTOG 1010 is a phase III trial evaluating radiation, paclitaxel, carboplatin with or without trastuzumab in locally advanced HER2 overexpressing esophageal and gastroesophageal junction adenocarcinoma (NCT01196390). The primary end point is DFS. The trial completed accrual and the estimated final data collection for the primary outcome is August 2018.
In the postoperative setting there is a Turkish phase II, single arm, open-label study called TOXAG evaluating the safety and efficacy of the combination oxaliplatin, capecitabine, trastuzumab, and radiation as adjuvant treatment of patients with curatively resected HER2-positive gastric or gastroesophageal junction cancer. Patients will receive concurrent chemotherapy with radiation. Trastuzumab will be continued after radiation for a total of 12 months. The expected completion date is December 2017.
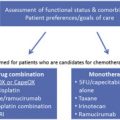
Advanced disease
Trastuzumab
Trastuzumab, a monoclonal antibody directed against HER2 receptor, is the first targeted agent approved for the treatment of advanced EGC. The randomized phase III trial that led to the approval of was published in 2010 and is known as the ToGA (Trastuzumab for Gastric Cancer) trial. At that time, previous preclinical models of HER2-overexpressing human gastric cancer xenografts suggested that trastuzumab had some activity when used alone but results were better when it was combined with different chemotherapy drugs. The agents most active were a fluorinated pyrimidine or cisplatin, or both (see Table 1 ).
In the ToGA study, patients with advanced HER2-positive adenocarcinoma of the stomach or gastroesophagic junction, without previous treatment of metastatic disease, were randomized to receive trastuzumab at an initial dose of 8 mg/kg intravenously followed by 6 mg/kg intravenously every 3 weeks plus capecitabine, or fluorouracil and cisplatin, or chemotherapy alone. The primary end point was OS. In total, 594 patients were included from centers in Asia, Central and South America, and Europe. The trial was closed after the second interim analyses. The median OS in the group treated with trastuzumab was 13.8 months compared with 11.1 months in the control group (hazard ratio [HR], 0.74; 95% CI, 0.60–0.91; P = .0046); progression-free survival was 6.7 versus 5.5 months (HR, 0.71; 95% CI, 0.59–0.85; P = .0002), and overall response rate was 47% versus 35% ( P = .0017), respectively. The side effects were comparable between both study groups and grade 3 or 4 toxicities included mainly neutropenia, anemia, nausea, anorexia, and vomiting. Cardiac events, a major concern in patients with breast cancer especially when trastuzumab and anthracyclines are part of the treatment, were rare in this study and cardiac toxicity rate was less than 1% and not different from patients treated with chemotherapy alone.
The FISH test is generally considered positive for HER2 amplification when the HER2/CEP17 ratio is greater than or equal to 2.0, which was one criterion for HER2 positivity on the TOGA trial. A preplaned exploratory analyses showed that the greatest OS benefit with trastuzumab was seen in patients who had tumors with high HER2 protein expression by IHC (HR, 0.65; median OS, 16.0 vs 11.8 months). More recently, a Spanish study analyzed data on 66 patients with HER2 amplification who received anti-HER2 therapy associated with chemotherapy and correlated with clinical outcomes. In this study, the mean HER2/CEP17 ratio of 4.7 (95% CI, 4.0–6.8) was the optimal cutoff value for discriminating between sensitive and refractory patients treated with trastuzumab-based chemotherapy. This criterion, however, has not been validated in clinical practice.
Based on these results, the Food and Drug Administration approved this combination of trastuzumab, cisplatin, and a fluoropyrimidine for the first-line treatment of patients with HER2-positive advanced adenocarcinoma of the esophagogastric junction and the stomach.
Lapatinib
Lapatinib is a small-molecule tyrosine kinase inhibitor that targets HER2 and epidermal growth factor receptor (EGFR). Lapatinib is approved by the US Food and Drug Administration for use in metastatic HER2-positive breast cancer that is refractory to trastuzumab, in combination with capecitabine. The role of lapatinib in the treatment of advanced EGC has been investigated in two phase III studies.
The phase III LOGiC (Lapatinib in Combination With Capecitabine Plus Oxaliplatin in Human Epidermal Growth Factor Receptor 2–Positive Advanced or Metastatic Gastric, Esophageal, or Gastroesophageal Adenocarcinoma) trial studied the use of capecitabine and oxaliplatin with or without lapatinib in the first-line setting for HER2-positive esophagogastric adenocarcinoma with the primary end point of OS. A total of 545 patients were included and the median follow-up was 23 months. Although the final results are negative because there was no statistically significant improvement in OS (12.2 vs 10.5 months; HR, 0.91; 95% CI, 0.73–1.12; P = .3492), the response rate was higher in the lapatinib arm (53% vs 39%; P = .0031). More patients experienced serious toxicities in the lapatinib arm compared with chemotherapy only, including diarrhea, nausea, vomiting, and fatigue.
In the phase III TyTAN study (Lapatinib Plus Paclitaxel vs Paclitaxel Alone in the Second-Line Treatment of HER2-Amplified Advanced Gastric Cancer in Asian Populations) enrolled patients with HER2-positive EGC after progression on a first-line therapy. The primary end point was OS and they included 261 patients. Median OS was 11.0 months with lapatinib plus paclitaxel versus 8.9 months with paclitaxel alone. This difference was not statistically significant (HR, 0.84; 95% CI, 0.64–1.11; P = .1044). Again, there was an increase in the response rate in the lapatinib arm (27% vs 9%).
In breast cancer, HER2 amplification by FISH is a stronger predictor of benefit from lapatinib than HER2 overexpression by IHC. Similarly, in the TyTAN study, those who were treated with lapatinib and whose tumors were strongly HER2 FISH-positive/IHC 3+ had better OS (HR, 0.59; P = .0176).
Trastuzumab Emtansine
Trastuzumab emtansine (T-DM1), an antibody-drug conjugate consisting of the trastuzumab linked to the cytotoxic agent emtansine, was explored in the second-line treatment of HER2-positive EGC after trastuzumab progression in the GATSBY trial that was presented in 2016.
The GATSBY trial was initially designed as a three-arm randomized, phase II/III global study of T-DM1 versus a taxane drug in patients with HER2-positive unresectable EGC who had received first-line treatment including a fluoropyrimidine plus platinum agent with or without a HER2-targeted therapy. At first, patients were randomized to T-DM1 3.6 mg/kg every 3 weeks, T-DM1 2.4 mg/kg weekly, or the physician’s choice of paclitaxel or docetaxel. After an independent data monitoring committee selected T-DM1 weekly for further study the patients were then randomized to either T-DM1 weekly or a taxane. The primary end point was OS. A total of 415 patients were randomized. The results showed a median OS of 8.6 months for the group treated with a taxane and 7.9 months for the group treated with T-DM1. Objective response rates were 19.6% and 20.6% in the groups treated with a taxane and T-DM1 respectively. Based on these results, T-DM1 showed no benefit in the second-line treatment when compared with a taxane and therefore no standard exists for HER2-directed therapy after trastuzumab progression.
Pertuzumab
Pertuzumab in an antibody that binds to the extracellular domain of HER2 at a different epitope than trastuzumab leading to a more complete blockade of HER2-mediated signal transduction when used in combination with trastuzumab. The JACOB trial explored first-line pertuzumab with trastuzumab and chemotherapy in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer, and results are expected in 2016 ( NCT01774786 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree