The treatment of locally advanced esophageal cancer is controversial. For patients who are candidates for surgical resection, multiple prospective clinical trials have demonstrated the advantages of neoadjuvant chemoradiation. For patients who are medically inoperable, definitive chemoradiation is an alternative approach with survival rates comparable to trimodality therapy. Although trials of dose escalation are ongoing, the standard radiation dose remains 50.4 Gy. Modern radiotherapy techniques such as image-guided radiation therapy with motion management and intensity-modulated radiation therapy are strongly encouraged with a planning objective to maximize conformity to the intended target volume while reducing dose delivered to uninvolved normal tissues.
Key points
- •
General guidelines for treatment of locally advanced esophageal cancer include both preoperative and nonoperative approaches, predicated upon resectability, histology, and location.
- •
In patients with resectable disease who are medically fit for this procedure, pre-operative chemoradiation to 50.4 Gy, with consideration of a lower dose (41.4 Gy) based on the CROSS trial, is recommended.
- •
In non-operative patients, definitive chemoradiation to 50.4 Gy is standard; however, enrollment of these patients on dose-escalation or other protocols is encouraged.
- •
Clinicians should make use of all available imaging and diagnostic modalities to delineate tumor and involved lymphadenopathy.
Overview
Locally-advanced esophageal cancer (LAEC) is broadly defined as American Joint Commission on Cancer, version 7, clinical stage III or IVA (T3-4 and/or N+) disease. Therapeutic approaches commonly include preoperative concurrent chemotherapy plus radiation (chemoradiation) or definitive chemoradiation alone. There is controversy regarding the ideal therapeutic approach to this disease. The US Patterns of Care Study offers a historical perspective. A total of 414 patients (51%: adenocarcinoma and 49%: squamous cell carcinoma [SCC]) received radiation therapy as part of definitive or adjuvant management at 59 institutions from 1996 to 1999. Overall, patients who received chemoradiation followed by surgery had a significant decrease in locoregional recurrence (hazard ratio [HR], 0.40, P <.0001) and survival improvement (HR, 0.32, P <.001) compared with those who did not undergo surgery. A similar significant decrease in locoregional recurrence (HR, 1.36, P = .01) and survival (HR 1.32, P <.03) was seen in those patients who received their care at large radiation oncology centers (treating ≥500 new patients with cancer per year) compared with small centers (treating <500 new patients with cancer per year).
In the current review, the authors discuss the management of inoperable esophageal cancer and review neoadjuvant chemoradiation, perioperative chemotherapy, and radiotherapy techniques.
Overview
Locally-advanced esophageal cancer (LAEC) is broadly defined as American Joint Commission on Cancer, version 7, clinical stage III or IVA (T3-4 and/or N+) disease. Therapeutic approaches commonly include preoperative concurrent chemotherapy plus radiation (chemoradiation) or definitive chemoradiation alone. There is controversy regarding the ideal therapeutic approach to this disease. The US Patterns of Care Study offers a historical perspective. A total of 414 patients (51%: adenocarcinoma and 49%: squamous cell carcinoma [SCC]) received radiation therapy as part of definitive or adjuvant management at 59 institutions from 1996 to 1999. Overall, patients who received chemoradiation followed by surgery had a significant decrease in locoregional recurrence (hazard ratio [HR], 0.40, P <.0001) and survival improvement (HR, 0.32, P <.001) compared with those who did not undergo surgery. A similar significant decrease in locoregional recurrence (HR, 1.36, P = .01) and survival (HR 1.32, P <.03) was seen in those patients who received their care at large radiation oncology centers (treating ≥500 new patients with cancer per year) compared with small centers (treating <500 new patients with cancer per year).
In the current review, the authors discuss the management of inoperable esophageal cancer and review neoadjuvant chemoradiation, perioperative chemotherapy, and radiotherapy techniques.
Definitive therapy in unresectable locally advanced esophageal cancer
Radiation Monotherapy
In LAEC, radiation therapy alone should be reserved for palliation or for patients who are medically unfit to receive concurrent chemotherapy. The 5-year survival rate for patients treated with conventional doses of radiation therapy alone is suboptimal. In the landmark Radiation Therapy Oncology Group (RTOG) 85-01 trial in which patients were randomized to receive either 64 Gy at 2 Gy/d or chemoradiation, all patients who received radiation alone were all dead of disease by 3 years. Shi and colleagues reported a 33% 5-year survival rate with the use of late course accelerated fractionation to a total dose of 68.4 Gy.
Primary radiation is more successful in patients with cT1N0 disease. Sai and colleagues from Kyoto University treated 34 patients who were either medically inoperable or refused surgery with either external beam alone (64 Gy) or external beam (52 Gy) plus 8 to 12 Gy with brachytherapy. The 5-year results included 59% overall survival, 68% local relapse-free survival, and 80% cause-specific survival. Yamada and colleagues reported the results in a similar group of 63 patients treated with chemoradiation plus brachytherapy. The 5-year results included 66% overall survival, 64% disease-free survival, and 76% cause-specific survival.
Brachytherapy Boost
Brachytherapy can be delivered by low- or high-dose rates and has previously been used as a boost following external beam radiation therapy or chemoradiation. This technique is limited by the effective treatment distance. The primary isotope is Iridium 192 (Ir-192), which is usually prescribed to treat to a distance of 1 cm from the source. Therefore, as confirmed by pathologic analysis of treated specimens, any portion of the tumor that is greater than 1 cm from the source will receive a suboptimal radiation dose.
There does not appear to be an advantage of adding brachytherapy to external beam radiation. One series reported a local failure rate of 57% and a 5-year actuarial survival of 28% in 46 patients with stage T2-3N0-1M0 disease. Even in patients with earlier stage disease (clinical T1-2), brachytherapy likely does not offer an advantage. Yorozu and colleagues reported a local failure rate of 44% and a 5-year survival of 26%, and Pasquier and associates reported local failure of 23% and the 5-year survival of 36%. However, in an updated series by Ishikawa and colleagues, 59 patients with submucosal esophageal cancer received external beam followed by brachytherapy in a subset of 36 patients with either low-dose-rate Caesium 137 (17 patients) or high-dose-rate Ir-192 (19 patients). Patients selected to receive a brachytherapy boost had a significantly higher 5-year cause-specific survival (86% vs 62%, P = .04).
Chemoradiation plus brachytherapy was tested prospectively by the RTOG Trial 9207. A total of 75 patients with cancers of the thoracic esophagus (92% squamous cell, 8% adenocarcinoma) received the RTOG 8501 50-Gy chemoradiation regimen followed by a boost during cycle 3 of chemotherapy with either low-dose-rate or high-dose-rate intraluminal brachytherapy. Because of low accrual, the low-dose-rate option was discontinued, and the analysis was limited to patients who received the high-dose-rate treatment. High-dose-rate brachytherapy was delivered in weekly fractions of 5 Gy during weeks 8, 9, and 10. Several patients developed fistulas, and the fraction delivered at week 10 was discontinued. The complete response rate was 73%. With a median follow-up of only 11 months, local failure as the first site of failure was 27%. Acute toxicities were high. These acute toxicities included 58% grade 3, 26% grade 4, and 8% grade 5 (treatment-related death). The cumulative incidence of fistula was 18%/y and the crude incidence was 14%. Of the 6 treatment-related fistulas, 3 were fatal. Significant toxicity, combined with the lack of dramatic efficacy and the labor-intensive nature of brachytherapy, has resulted in limited interest in developing this technique further in esophageal cancer.
If brachytherapy is to be used, guidelines for esophageal brachytherapy published by the American Brachytherapy Society are available. For patients treated in the curative setting, brachytherapy should be limited to tumors 10 cm or less with no evidence of distant metastasis. Contraindications include tracheal or bronchial involvement, cervical esophagus location, or stenosis that cannot be bypassed. The applicator should have an external diameter of 6 to 10 cm. If chemoradiation is used (defined as 5-fluorouracil [5-FU]–based chemotherapy plus 45–50 Gy), the recommended doses of brachytherapy are 10 Gy in 2 weekly fractions of 5 Gy each for high-dose rate and 20 Gy in a single fraction at 4 to 10 Gy/h for low-dose rate. The doses should be prescribed to 1 cm from the source. Last, brachytherapy should be delivered after the completion of external beam and not concurrently with chemotherapy.
Definitive Chemoradiation
Although there are 6 randomized trials comparing definitive radiation therapy alone with chemoradiation, the only trial designed to deliver adequate doses of systemic chemotherapy with concurrent radiation therapy was the RTOG 85-01 trial reported by Herskovic and colleagues. As was common in the 1980s, most patients had SCC. Treatment included 4 cycles of 5-FU (1000 mg/m 2 /24 h × 4 days) and cisplatin (CDDP; 75 mg/m 2 , day 1). Radiation therapy (50 Gy at 2 Gy/d) was given concurrently with the first day of cycle 1 of chemotherapy. Cycles 3 and 4 of chemotherapy were delivered every 3 weeks rather than every 4 weeks. Only 50% of the patients finished all 4 cycles of the chemotherapy. The control arm was radiation therapy alone, albeit a higher dose (64 Gy) than the chemoradiation arm.
Patients treated with chemoradiation had a significant improvement in both median (14 months vs 9 months) and 5-year survival (27% vs 0%, P <.0001). The 8-year survival was 22%. Histology did not significantly influence the results. The 5-year survival was 21% for the 107 patients with SCC versus 13% of the 23 patients with adenocarcinoma, P = NS. Local failure (defined as local persistence plus recurrence) was also lower in the chemoradiation arm (47% vs 65%). Although African Americans had larger primary tumors of which all were SCC, there was no difference in survival compared with Caucasians.
Dose Escalation—2-Dimensional and 3-Dimensional Techniques
These promising results led to Intergroup 0122, a phase II trial of dose-escalated chemoradiation to 64.8 Gy delivered concurrently with CDDP and 5-FU. This regimen appeared to have acceptable toxicity and formed the experimental arm of INT 0123 (RTOG 9405). In this trial, patients selected for a nonsurgical approach were randomized to a slightly modified RTOG 85-01 chemoradiation regimen with 50.4 Gy versus the same chemotherapy with 64.8 Gy, based on INT 0122. As with RTOG 85-01, most patients (85%) had SCC.
There were several modifications to the original RTOG 85-01 chemoradiation arm. These modifications included using 1.8-Gy fractions to 50.4 Gy rather than 2-Gy fractions to 50 Gy, treating with 5 cm proximal and distal margins for 50.4 Gy rather than treating the whole esophagus for the first 30 Gy followed by a cone down with 5-cm margins to 50 Gy; cycle 3 of 5-FU/CDDP did not begin until 4 weeks following the completion of radiation therapy rather than 3 weeks, and last, cycles 3 and 4 of chemotherapy were delivered every 4 weeks rather than every 3 weeks. The trial opened in late 1994 and was closed in 1999 when an interim analysis revealed that it was unlikely that the high-dose arm would achieve a superior survival compared with the standard dose arm.
For the 218 eligible patients, there was no significant difference in median survival (13.0 months vs 18.1 months), 2-year survival (31% vs 40%), or local/regional failure and/or local/regional persistence of disease (56% vs 52%) between the high-dose and standard-dose arms. Although 11 treatment-related deaths occurred in the high-dose arm compared with 2 in the standard-dose arm, 7 of the 11 occurred in patients who had received 50.4 Gy or less.
An alternative approach to dose escalation is altered fractionation; this has been investigated in LAEC with modest results. Zaho and colleagues treated 201 patients with squamous cell cancer using 41.4 Gy followed by late-course accelerated hyperfractionation to 68.4 Gy. The results were similar to RTOG 85-01 (38% local failure and 26% 5-year survival). Choi and colleagues treated 46 patients with 5-FU/CDDP and twice-a-day radiation using a concurrent boost technique and reported a 37% 5-year survival. In addition, Lee and colleagues reported a trial of 102 patients with LAEC, limited to SCC, randomized to surgery alone versus preoperative therapy with 45.6 Gy (1.2 Gy twice a day) plus 5-FU/CDDP. There was no difference in median survival (28 vs 27 months). Thus, although these approaches may appear to be reasonable, there appears to be a significant increase in acute toxicity without any clear therapeutic benefit.
Dose Escalation—Intensity-Modulated Radiation Therapy and Protons
A criticism of many dose escalation trials in the definitive management of LAEC is the use of conventional 2-dimensional (2D) and 3-dimensional (3D) radiation techniques. Trials using newer techniques such as intensity-modulated radiation therapy (IMRT) may be able to deliver higher doses of radiation with a more tolerable toxicity profile. Multiple dosimetric studies comparing standard 3-D conformal radiotherapy (3D-CRT) and IMRT generally have found improved sparing of the heart, lung, or both using either static field or arc-based IMRT. The dosimetric advantages of IMRT has led multiple clinical centers to begin the routine use of IMRT in this disease. Retrospective analysis of these data does not suggest inferior outcome and may provide decreased toxicity versus non-IMRT treatment techniques. Investigators at MD Anderson Cancer Center reported the results of 676 patients with LAEC treated with either IMRT (263) or 3D-CRT (413). On multivariate analysis, IMRT was associated with improved survival ( P = .004), but not cancer-specific survival ( P = .86). The survival difference between 3D-CRT and IMRT was thought to be due to a higher level of cardiac ( P = .05) and unexplained deaths ( P = .003) in the 3D-CRT patients, suggesting that decreased cardiac dose may have a direct impact on patient outcome. Although this and other comparisons between 3D-CRT and IMRT in LAEC are retrospective, a randomized trial is unlikely; thus, the available data may represent the best comparison.
Another theoretic advantage of IMRT is the possibility of dose escalation. With the use of IMRT, a simultaneous integrated boost may be performed while maintaining commonly used lung and heart dosimetric constraints. Retrospective data from Zhang and colleagues suggest a positive correlation between radiation dose and locoregional control. This positive correlation has led to a phase I studying examining this approach in LAEC at MD Anderson Cancer Center. However, at this point, based on results of the INT 0123 trial, the standard dose of external beam radiation remains 50.4 Gy.
The use of proton therapy remains investigational.
Perioperative and Induction Chemotherapy
For locally advanced lower esophageal and gastroesophageal (GE) junction cancers, perioperative chemotherapy may be considered as a therapeutic option, although such an approach has not been validated for midthoracic or upper esophageal cancers. The UK Medical Research Council Adjuvant Gastric Infusional Chemotherapy (MAGIC) trial that showed perioperative epirubicin, CDDP, and 5-FU chemotherapy provides a 10% survival advantage when given in addition to definitive surgery. However, lower esophageal and GE junction cancers represented less than 25% of patients enrolled in the MAGIC trial with the vast majority of patients having gastric cancer, creating challenges for interpreting these data in esophageal cancer. Pathologic complete response rate, not reported in this trial, is generally relatively low with chemotherapy alone.
The ideal approach for lower esophageal cancer with either perioperative chemotherapy or trimodality therapy has been debated. Interpretation of the data is also challenging because these trials have primarily enrolled gastric cancer with only small numbers of lower esophageal cancer. Given the limited number of esophageal cancers limited to the lower esophagus in these perioperative chemotherapy trials, caution should be exercised in interpreting these data.
A potential advantage of neoadjuvant chemotherapy is the early identification of those patients who may or may not respond to the chemotherapeutic regimen being delivered concurrently with chemoradiation. Lordick and colleagues demonstrated that patients without a response to preoperative chemotherapy on fluorodeoxyglucose (FDG)-PET scan did not benefit from continuing this therapy and could be sent to immediate surgery without compromising survival. Ilson and colleagues have shown that the change in standardized uptake value (SUV) on FDG-PET scan during induction chemotherapy was able to predict which patients showed a response to subsequent chemoradiotherapy. Wieder and associates reported similar findings in 38 patients with squamous cell cancers. Although this approach is investigational, if the nonresponders can be identified early, changing the chemotherapeutic regimen may be helpful. However, in the context of induction chemotherapy before definitive chemoradiation, the data do not support its routine use. For example, Ruhstaller and colleagues report the outcomes from a phase II trial using CDDP/docetaxel followed by chemoradiation in unresectable LAEC. In this study, median survival was 16 months, with 29% of patients surviving long term, suggesting no benefit over chemoradiation alone. Recently, the Cancer and Acute Leukemia Group B (CALGB)/Alliance completed a randomized phase II trial using PET scan response to guide selection of chemotherapy to use during preoperative chemoradiotherapy, and results are expected in 2017.
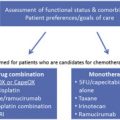
In summary, either perioperative chemotherapy or neoadjuvant chemoradiotherapy can be considered for lower and GE junction cancers. However, for patients who have positive margins or progress after induction chemotherapy, chemoradiotherapy should be considered. For patients with LAEC above the low esophagus and GE junction, neoadjuvant chemoradiotherapy remains the standard of care ( Table 1 ).
| Trial | N | Patients | Comparison | Concurrent Chemotherapy | Outcome | Comments |
|---|---|---|---|---|---|---|
| Urba et al, 1993 | 100 | Adenocarcinoma: 75% SCC: 25% | Arm 1: Surgery Arm 2: 45 Gy/1.5 Gy BID → Surgery | CDDP, 5-FU, and vinblastine | MS: 17.6 mo Arm 2 vs 16.9 mo Arm 1 (NS) LRF: 19% Arm 2 vs 42% Arm 1 ( P = .02) | Small study, required a >1 y improvement in MS to be significant |
| Walsh et al, 2012 | 113 | Adenocarcinoma: 100% | Arm 1: Surgery Arm 2: 40 Gy/2.67 Gy QD → Surgery | CDDP and 5-FU | MS: 16 mo Arm 2 vs 11 mo Arm 1 ( P = .01) | Low survival rate in Arm 1 compared with other trials |
| FFCD 8805/EORTC 40881 | 282 | SCC: 100% | Arm 1: Surgery Arm 2: 18.5 Gy/3.7 QD→ 2 wk break → 18.5 Gy/3.7 QD →Surgery | CDDP | MS: 18.6 for both arms (NS) DFS: 40% Arm 2 vs 28% Arm 1 ( P = .003) | Unconventional radiotherapy |
| TTROG/AGITG | 128 | Adenocarcinoma: 63% SCC: 35% | Arm 1: Surgery Arm 2: 30 Gy/3 Gy QD → Surgery | CDDP and 5-FU | MS: 22.2 mo Arm 2 vs 19.3 mo Arm 1 (NS) | R0 80% in Arm 2 vs 59% Arm 1 |
| Lee et al, 2004 | 101 | SCC: 100% | Arm 1: Surgery Arm 2: 45.6 Gy/1.2 Gy BID → Surgery | CDDP and 5-FU | MS: 28.2 mo Arm 2 vs 27.3 mo Arm 1 (NS) | 31% of patients in Arm 2 did not have a surgical resection |
| CALGB 9781 | 56 | Adenocarcinoma: 75% SCC: 25% | Arm 1: Surgery Arm 2: 50.4 Gy/1.8 Gy QD → Surgery | CDDP and 5-FU | MS: 53.8 mo Arm 2 vs 21.5 mo Arm 1 ( P = .002) | Closed early due to poor accrual |
| CROSS | 366 | Adenocarcinoma: 75% SCC: 23% | Arm 1: Surgery Arm 2: 41.4 Gy/1.8 Gy QD → Surgery | Carboplatin and paclitaxel | MS: 49.4 mo Arm 2 vs 24 mo Arm 1 ( P = .003) | R0: 93% in Arm 2 vs 69% in Arm 1 |
Tracheoesophageal Fistula
A malignant tracheoesophageal (TE) fistula is an unfavorable prognostic feature, and its management deserves special attention. Although the survival of such patients is low, occasionally they may have long-term survival. Historically, the use of radiation therapy was contraindicated due to the concern of exacerbating the fistula as the tumor responded. However, some data suggest that this is not the case. At the Mayo Clinic, 10 patients with a malignant TE fistula received 30 to 66 Gy and their median survival was 5 months. None of the patients experienced an enlarging or more debilitating fistula following radiation. Rueth and colleagues showed improved survival with a palliative course of radiation compared with stent placement alone (3.3 vs 1 month) and no significant difference in complications. Finally, in a series of 24 patients with TE fistulae, Muto and colleagues found a 71% TE fistula closure rate following chemoradiation, with a median survival time of 6.7 months. The data, albeit limited, suggest that radiation does not necessarily increase the severity of a malignant TE fistula, and it is not a contraindication to its use. However, given the overall poor prognosis of this subset of patients, the impact on outcome is not clear.
Trimodality therapy
Preoperative Chemoradiation
There are 7 randomized trials comparing preoperative combined modality therapy with surgery alone in patients with clinically resectable disease, the most recent being the Chemoradiotherapy for Oesophageal Cancer followed by Surgery Study (CROSS).
The CROSS trial randomized 366 patients with LAEC (75% adenocarcinoma, 23% SCC) to receive either neoadjuvant chemoradiation with 41.4 Gy and carboplatin/paclitaxel followed by surgical resection versus surgical resection alone. In this trial, median survival was effectively doubled by the addition of chemoradiation (49.4 vs 24 months, P = .003). Improved survival was seen in both adenocarcinoma and SCC, although the magnitude was slightly greater in SCC. The R0 resection was 93% in the chemoradiation arm, compared with 69% in the surgery alone arm ( P <.001). Despite concerns that a lower radiation dose combined with carboplatin and paclitaxel may not be as effective, the pathologic complete response (pCR) rate was 29%. The pCR rate and survival in the CROSS trial is comparable to most previous trials and retrospective reviews. In addition, no significant difference in perioperative complications was seen between treatment arms.
Before the publication of the CROSS trial, the role of preoperative chemoradiation was controversial. The first 6 trials (Urba, Walsh, EORTC, Australasian, Korea, and CALGB 9781 ) had limited patient numbers and heterogeneous treatment regimens, and in some, the dose of radiation was insufficient based on a dose response analysis by Geh and colleagues. Despite all of these limitations, a meta-analysis did suggest a survival benefit. However, with the publication of the CROSS trial, the standard of care for patients with locally advanced but medically resectable adenocarcinoma of the esophagus is now preoperative chemoradiation.
Necessity for Surgery Following Chemoradiation
Because of the known response of LAEC to chemoradiation as well as the significant morbidity of an esophagectomy, questions arise as to the necessity of this procedure. Two randomized trials examine whether surgery is necessary after chemoradiation. In the Federation Francaise de Cancerologie Digestive (FFCD) 9102 trial, 445 patients with clinically resectable T3-4N0-1M0 SCC or adenocarcinoma of the esophagus received initial chemoradiation. The vast majority of patients (90%) in this trial had squamous cancers. Patients initially received 2 cycles of 5-FU, CDDP, and concurrent radiation (either 46 Gy at 2 Gy/d or split course 15 Gy weeks 1 and 3). The 259 patients who had at least some evidence of clinical response were then randomized to surgery versus additional chemoradiation, which included 3 cycles of 5-FU, CDDP, and concurrent radiation (either 20 Gy at 2 Gy/d or split course 15 Gy). There was no significant difference in 2-year survival (34% vs 40%, P = .56) or median survival (18 months vs 19 months) in patients who underwent surgery versus additional chemoradiation. These data suggest that for patients who initially respond to chemoradiation, they should complete chemoradiation rather than stop and undergo surgery. Using the Spitzer index, there was no difference in global quality of life; however, a significantly greater decrease in quality of life was observed in the surgery arm during the postoperative period (7.52 vs 8.45, P <.01, respectively). In a separate trial that compared with split course radiation, patients who received standard course radiation had improved 2-year local relapse-free survival rates (77% vs 57%, P = .002), but no significant difference in overall survival (37% vs 31%).
The German Oesophageal Cancer Study Group compared preoperative chemoradiation followed by surgery versus chemoradiation alone. In this trial, 172 eligible patients less than 70 years old with uT3-4N0-1M0 SCC were randomized to preoperative therapy (3 cycles of 5-FU, leucovorin, etoposide, and CDDP, followed by concurrent etoposide, CDDP, plus 40 Gy) followed by surgery versus chemoradiation alone (the same chemotherapy but the radiation dose was increased to 60–65 Gy ± brachytherapy). The pCR rate was 33%. Although there was a decrease in 2-year local failure (36% vs 58%, P = .003), there was no significant difference in 3-year survival (31% vs 24%) for those who were randomized to preoperative chemoradiation followed by surgery versus chemoradiation alone.
Despite the above data, the current standard of care is to perform esophagectomy following chemoradiation in patients who can tolerate this approach. However, it is known that a subset of patients will have a complete response to chemoradiation. Furthermore, it is known that patients with pCR have improved survival. Data from both Berger and colleagues and Rohatgi and colleagues suggest that patients who achieve a pCR had an improvement in survival compared with those who do not (5 year: 48% vs 15%, and median: 133 months vs 34 months, respectively). In these patients, surgical resection may not be necessary and has led to the concept of “selective” surgery after preoperative chemoradiation.
Swisher and colleagues have also reported a retrospective analysis of patients who underwent a salvage compared with a planned esophagectomy. The operative mortality was higher in those who underwent salvage versus planned surgery (15% vs 6%), but there was no difference in survival (25%). However, only 13 patients were identified who had salvage, limiting the broad interpretation of these findings. However, a recent phase II trial, RTOG 0246, prospectively examined the approach of preoperative paclitaxel/CDDP and 50.4 Gy followed by selective surgery in patients with either residual disease or recurrent disease in the absence of distant metastasis. In this trial of 43 patients with LAEC, 21 patients required surgical resection after chemoradiation due to residual (17 patients) or recurrent (3 patients) disease. This approach led to a 1-year overall survival of 71%, lower than the predetermined survival rate (77.5%).
For patients with adenocarcinoma, there is substantial evidence that esophagectomy is critically important for oncologic outcomes. Although definitive chemoradiotherapy with CDDP and 5-FU in RTOG 8501 had an overall survival of 26%, nearly all of these survivors had squamous histology and only 1 of 23 patients with adenocarcinoma was alive at 5 years. The importance of definitive surgical management for adenocarcinoma is further corroborated by findings in the CROSS trial showing that there was a pCR rate of only 23% for adenocarcinoma compared with 49% for SCC. Thus, for patients who receive concurrent chemoradiotherapy either with carboplatin and paclitaxel or with CDDP and 5-FU, there is no clear evidence that esophagectomy can be omitted for adenocarcinoma.
Evaluation of Response to Chemoradiation
To further pursue the selective surgical approach as a treatment modality, it will be critical to establish the definition of an adequate response. However, the ability to predict a pCR before surgery is variable. A multivariate analysis by Gaca and colleagues reported that posttreatment nodal status ( P = .03) but not the degree of primary tumor response predicted disease-free survival.
Current available imaging modalities and/or postchemoradiation biopsies are also of limited value in predicting a pCR. Bates and colleagues noted a 41% false-negative rate with preoperative endoscopy and biopsy. Jones and colleagues reported that computed tomography (CT) had a sensitivity of 65%, a specificity of 33%, a positive predictive value of 58%, and a negative predictive value of 41% in evaluating pathologic response after preoperative chemoradiation. Many studies show that endoscopic ultrasound (EUS) performed after chemoradiation is a suboptimal predictor of complete response because of the inability to distinguish postirradiation fibrosis and inflammation from residual tumor. Reported accuracy is generally at or less than 50%. For example, Sarkaria and colleagues found that in 165 patients a negative endoscopic biopsy was not a useful predictor of a pCR after chemoradiation (31% negative predictive value), final nodal status, or overall survival.
The value of FDG-PET for staging after chemoradiation is unclear. Several studies of patients with esophageal cancer show that an early decrease in FDG uptake after chemotherapy can predict clinical response. In addition, multiple studies have evaluated the ability of FDG-PET to predict a pCR following chemoradiation. Flamen and colleagues evaluated the predictive value of PET after chemoradiation in patients receiving preoperative treatment. The sensitivity and positive predictive value of PET for identifying a pCR were 67% and 50%, respectively. Both false-positive PET findings (residual FDG activity in an area of intense inflammatory activity on histopathologic analysis) and false-negative findings occurred at the primary tumor site. Vallbohmer and colleagues treated 119 patients with preoperative chemoradiation and reported a nonsignificant association between major responders and FDG-PET results ( P = .056). There was no clear SUV threshold that predicted response. The inflammatory effect of chemoradiation as well as a lack of standardization of FDG-PET protocols and techniques and definitions of a pathologic response may be responsible for the variation in results. Thus, although most studies investigating the role of posttreatment FDG-PET in evaluating pCR found some correlation between the 2, a wide array of SUV threshold values and a lack of specificity preclude its use as a surrogate marker of pCR.
Biomarkers of Response
Because of the marginal results of using clinical variables to predict and assess pCR following chemoradiation, attention has been shown to the use of pathologic or molecular markers to this end. Studies have linked tumor lymphocytic infiltration as well as apoptotic index with response to chemoradiation. Additional studies have linked a large number of proteins and genes involved in a wide array of signaling cascades with response to chemoradiation. Examples include alterations in diverse signaling cascades involving PI3 kinase, p53, EGFR, and HIF-1α. Unfortunately, the vast majority of these studies lack validation and the specificity required to be used clinically. One recent study generated a micro-RNA signature to predict pCR from LAEC tumors in 52 patients treated uniformly with chemoradiation. This signature was then validated in a separate cohort of 72 patients treated similarly. When combined with clinical stage, the area under the curve for pCR was 0.77 (p = 2 × 10 −41 ). These validated data argue for further investigation, possibly within the context of a clinical trial.
The human epidermal growth factor receptor 2 (Her2) is expressed in 20% to 30% of patients, where it is thought to contribute to tumor progression and therapeutic resistance. Initially, Her2-directed therapy was used in metastatic esophageal cancer in the Trastuzumab for Gastric Cancer trial for Her2 overexpressing tumors where traztuzumab provided a modest, but significant survival benefit. However, the utility of Her2 as a predictive biomarker for chemoradiotherapy in esophageal cancer is under investigation. The current NSABP, RTOG, GOG (NRG) Oncology/RTOG 1010 trial evaluated neoadjuvant concurrent carboplatin and paclitaxel ± traztuzumab (concurrent and maintenance) followed by surgical resection for Her2 overexpressing LAEC. The study is completed and the results are pending. Given the known cardiac toxicity of traztuzumab and potential synergistic toxicity with thoracic radiation therapy, it is not recommended to offer concurrent traztuzumab with cytotoxic chemotherapy and radiation off-protocol until results of NRG/RTOG 1010 are available.
Palliative Radiation Therapy
Despite advances in oncologic treatments for LAEC, the vast majority of patients will develop locoregional recurrence and/or metastatic disease. For patients with incurable esophageal cancer, systemic chemotherapy is the mainstay of therapy. However, locally advanced esophageal primary tumors can produce symptoms including dysphagia, chest pain, and bleeding. For such situations, radiation is a potent and effective palliative therapy. Most patients treated with radiation therapy will experience subjective relief of dysphagia from radiation therapy. Gastrointestinal bleeding is also effectively palliated by radiation therapy that may facilitate systemic therapy by reducing transfusion requirements. Although hypofractionated palliative radiation regimens have been used for other sites, hypofractionation can produce acute nausea and esophagitis. In the palliative setting, regimens, such as 30 Gy in 10 fractions or 37.5 Gy in 15 fractions, improve symptoms without excess toxicity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree