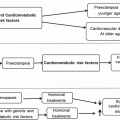
Recommended
Ineffective or harmful
Uncertain effect
EPA and DHA from oily fish or fish-oil supplements
Hormone therapy and selective estrogen receptor modulators (including soy isoflavones)
Vitamin D
Plant stanols and sterols
Antioxidant supplements (including vitamins E, C, and beta-carotene)
α-Linolenic acid
Folic acid in post-childbearing years (with or without vitamins B6 and B12)
Resveratrol
Dietary Factors with Demonstrated Benefit
Eicosapentaenoic Acid and Docosahexaenoic Acid
Epidemiological studies have revealed that cultures that traditionally consume a lot of fish, including Inuits in Greenland and Alaska and Japanese living in fishing villages, have dramatically lower rates of heart disease than cultures that do not (reviewed in [27]). Very long-chain n-3 PUFAs (also known as omega-3 PUFAs) are posited to be responsible for this dietary pattern effect. They are primarily found in oily fish, and EPA and DHA are the two most abundant forms. These n-3 PUFAs have been shown in diverse studies to have many cardioprotective effects, including decreases in plasma triglycerides [28], resting heart rate [29], and blood pressure [30]. They appear to be antiarrhythmic and thus may be particularly beneficial for preventing sudden cardiac death (SCD) (reviewed in [31]).
In 1989, the Diet and Reinfarction Trial (DART) examining myocardial reinfarction in men revealed that of three major dietary recommendations (reduction in fat, increase in fatty fish, and increase in cereal fiber), only fatty fish reduced mortality [32]. Ten years later, results of the large Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardio (GISSI)-Prevenzione study demonstrated that dietary supplementation with n-3 PUFAs in capsule form (but not vitamin E) in recent myocardial infarction (MI) survivors reduced fatal cardiac events, nonfatal MI, and stroke by 10–15 % after 3.5 years [33]. There was up to a 45 % reduction in SCD. A subsequent publication stratifying the results by follow-up time showed that protection against SCD was evident as early as 4 months [34]. However, only 15 % of the GISSI participants were female and it was not placebo-controlled. The Japan EPA Lipid Intervention Study (JELIS), involving over 18,000 hypercholesterolemic Japanese patients with or without previously diagnosed coronary artery disease (CAD), explored the effect of administration of highly purified EPA in combination with statin therapy compared to statins alone in a 5-year follow-up [35]. Major coronary events were reduced by 19 % in the EPA group, but there was no difference in SCD, unlike the GISSI trial. However, there are several caveats. There was also no true placebo group in this trial, 70 % of the participants were women even though Japanese women have a much lower incidence of CAD than Japanese men, and the Japanese population consumes on average five times more fish than other countries (suggesting that perhaps the effects on SCD are saturable). This was also a study of just EPA rather than EPA and DHA.
There are relatively few studies focused on women and the effects of EPA and DHA. The handful of randomized controlled trials that are devoted to women typically have few participants and are of short duration [36–39]. One small-scale study found that a fish-oil concentrate lowered serum triacylglycerides by 26 % in postmenopausal women after 28 days of treatment [39]. The NHS found protective effects of high fish consumption (≥5 times per week), particularly for CHD deaths, after 16 years of tracking women who were healthy at baseline [18]. The protective effects in preventing CHD were particularly strong for diabetic women (RR = 0.36 for women who consumed fish ≥5 times per week compared to women who seldom did [40]). While striking, large randomized placebo-controlled trials in women with long-term follow-up are needed to support the observations of the prospective trials.
There are several issues awaiting resolution. First, the optimal dosage is still somewhat unclear. Prospective studies mentioned above have noted a dose–response for fish intake in CVD protection. The AHA currently recommends 2 servings of oily fish per week or, secondarily, ~0.5–1 g of EPA and DHA per day from supplements [2, 25]. Some studies have shown triacylglyceride-lowering effects with larger doses, but it is recommended that a physician be consulted for advice on high-dose treatment [41, 42]. The efficacy of whole fish versus supplements is also debated [42]. While both fish consumption and supplementation with fish oil or EPA and DHA have been effective, it remains to be determined whether supplements can fully recapitulate the benefits of whole fish. This may be particularly relevant when whole fish is incorporated into the diet as a substitute for other animal protein sources that may be high in saturated fat. Finally, there is controversy over whether naturally derived or highly purified supplements are preferable [35, 41]. There are concerns about environmental toxins, such as methylmercury, dioxins, and PCBs, in natural sources (both whole fish and perhaps in even higher concentrations in fish-oil capsules). Other concerns include overfishing and access to/affordability of fresh fish. Considering the strong evidence for the benefits of EPA and DHA, answering these questions is important.
Plant Stanols and Sterols
High levels of serum cholesterol, particularly low density lipoprotein (LDL) cholesterol, are correlated with increased risk of CVD. It has been estimated that a 10 % reduction in total serum cholesterol would decrease the incidence of heart disease by >30 % for both men and women [43]. Serum cholesterol levels can be lowered by blocking cholesterol synthesis but also by interfering with cholesterol absorption. Phytosterols are plant-derived sterols structurally related to cholesterol. They can decrease both total and LDL cholesterol by reducing absorption and increasing elimination of cholesterol [44]. Although phytosterols have been added to hypercholesterolemic patients’ diets since the 1950s [45], and consumption typically lowers total cholesterol by ~10 %, focus has now shifted to the synthetic derivative sitostanol.
Sitostanol is a more potent cholesterol-lowering compound than sitosterol [46] and can easily be incorporated into foods, mainly in the form of spreads. In a 1-year randomized, double-blind study of 153 mildly hypercholesterolemic men and women, sitostanol reduced total serum cholesterol 10.2 % and LDL cholesterol by 14.1 %. Triglyceride and high density lipoprotein (HDL) cholesterol levels were unaffected [47]. In a small study of postmenopausal MI survivors, 2–3 months treatment with sitostanol ester margarine lowered total cholesterol 13 % and LDL cholesterol 20 % [48]. Sitostanol was also effective at lowering cholesterol even when women were taking statins to inhibit cholesterol synthesis. Although there are many trials incorporating sterols and stanols as part of the intervention protocol, they are mostly small-scale and relatively short-term and focus primarily on blood lipid profiles as endpoints [49]. It would be interesting to see the results of a long-term, adequately powered study on CVD incidence and mortality in women.
Dietary Factors with Possible Adverse Effects
Isoflavones
CVD risk in women increases after menopause, but estrogen can improve cholesterol levels and vascular tone, leading to the hypothesis that HRT could counteract the increased postmenopausal CVD risk. Alarmingly, HRT actually further increases risk [50], although the study design of waiting a number of years postmenopause to initiate HRT has been raised as a confounding factor [51]. Nonetheless, alternative therapies with estrogenic molecules have been pursued to lower the postmenopausal risk of CVD in the hopes of avoiding the negative consequences associated with HRT. Phytoestrogens are nonsteroidal plant-derived compounds with both estrogenic and antiestrogenic properties as well as receptor tyrosine kinase inhibitory effects and antioxidant properties. The phytoestrogens genistein and daidzein belong to the isoflavone family and are very abundant in soy. A low incidence of CVD has been noted in populations that consume a lot of soy-based foods, such as countries in the Pacific Rim. Certain studies have suggested that replacing animal protein with soy protein in the diet can lower blood lipid levels [52]. In 1999, a randomized trial of 156 mildly hypercholesterolemic men and women compared the effect of 9-week consumption of different isoflavone doses (prepared by adding back isoflavones to isoflavone-stripped soy protein) to casein, which is isoflavone-free [53]. At the highest isoflavone levels (62 mg/day), total cholesterol and LDL cholesterol were reduced by 4 % and 6 %, respectively. The effect was ascribed to isoflavones, although they did not compare to isoflavone-stripped soy protein without any added isoflavones or consider isoflavones either in the absence of protein or added instead to casein. Other studies have noted that, while soy protein can be effective in lipid lowering, isolated isoflavones typically are not, nor is isoflavone-stripped soy protein [54, 55]. This is also true in studies focused on postmenopausal women [56–60]. This discrepancy is still not completely understood, although the possibility remains that the soy protein effect is simply a misinterpretation of the effect of animal protein reduction. It has also been suggested that soy protein and isoflavones may somehow synergize, rendering either impotent without the other. To date, most isoflavone intervention studies have lacked the required power to detect benefits or risks of supplemental isoflavone intake. In addition, effects of estrogenic compounds in postmenopausal women may be sensitive to timing of intervention. In light of this accumulation of research, the AHA currently recommends against isoflavone dietary supplementation due to the unproven effects in CVD prevention and potential adverse effects in other disease settings [26]. They are also somewhat less enthusiastic about the impact of the potential cardiovascular benefits of soy protein than they were in 2000 [61]. The United States Food and Drug Administration currently allows labels of soy-containing foods to state that they may reduce the risk of heart disease, although they were cautious in their 1999 ruling to not specifically include isoflavones in the statement (Federal Register 64 FR 57699).
Antioxidant Supplements
It has been hypothesized that oxidative stress leads to CVD, among other chronic diseases [62]. The rationale is that free radicals can lead to LDL oxidation, lipid peroxidation, and DNA damage, culminating in CVD. However, whether free radicals are a consequence or a cause of CVD remains a subject of debate [63, 64]. Numerous observational and epidemiological studies have noted that diets high in fruits and vegetables are associated with decreased rates of CVD [65]. As fruits and vegetables are rich sources of antioxidants, which can scavenge free radicals, many trials have studied whether increased consumption of antioxidant vitamins (from the diet or in the form of dietary supplements) can prevent primary or secondary CVD. These include, but are not limited to, vitamins C and E and beta-carotene.
Vitamin C is the predominant circulating water-soluble antioxidant in humans. Although it has strong antioxidant properties, it can also act as a pro-oxidant under certain conditions [66]. Two prospective studies in women have reported effects of supplemental vitamin C, although not dietary vitamin C. The NHS found a modest protective effect of supplemental vitamin C in women in the highest quintile for intake as determined by FFQs [22]. However, these women also had healthier lifestyles overall, leading to moderate confounding. The Iowa Women’s Health Study tracked women at higher CVD risk due to diabetes for 15 years [67]. A FFQ similar to the NHS was used here, although only one FFQ was administered at baseline. They also found a supplemental vitamin C effect, but, contrary to the NHS, women in the highest quintile compared to the lowest were at increased risk of CVD mortality (RR = 1.84), CAD (RR = 1.91), and stroke (RR = 2.57). Rates of cardiovascular death in nondiabetic subjects were unaffected by vitamin C supplementation. Only one randomized trial, the Women’s Antioxidant Cardiovascular Study (WACS), looked at controlled administration of vitamin C supplements (as well as vitamin E and β-carotene, each individually assigned) for 9.4 years in at-risk populations of women [68]. They found no beneficial or harmful effect of any of these antioxidant supplements on CVD prevention.
Vitamin E is a class of lipid-soluble antioxidants comprised of eight different isomers: four tocotrienols and four tocopherols, including α (the major isoform in human plasma and tissue) and γ (the major isoform in food). It is a major constituent of LDL particles, where it presumably protects lipid and protein components from oxidative damage. Consequently, vitamin E supplementation has been studied in numerous CVD prevention trials. The Women’s Health Study was a randomized controlled trial that studied vitamin E supplementation for over 10 years in healthy women ≥45 years old [69]. Overall, there was no significant effect on the combined primary endpoint of major cardiovascular events. However, there was a significant 24 % reduction in the secondary endpoint of cardiovascular death. Moreover, subgroup analysis revealed a 26 % decrease in the primary endpoint among women older than 65. The prospective NHS also suggested a 41 % decrease among long-term vitamin E supplement users [21], although this was self-reported usage. Interestingly, a meta-analysis that pooled prospective studies found that high dietary intake of vitamin E, though not supplemental, was protective for women (24 % reduction) but not for men [70]. The Iowa Women’s Health Study also found a protective effect associated with high dietary vitamin E (RR = 0.38) but not supplemental in postmenopausal women [71]. The WACS, as mentioned above, found no protective effect for at-risk women [68]. Many other trials with both sexes participating have found no protective effect of vitamin E, including the GISSI-Prevenzione [33], the Primary Prevention Project [72], and the Heart Outcomes Prevention Evaluation (HOPE) trial [73]. Disturbingly, in some long-term, high-dose (≥400 IU/day) trials, harmful effects have been observed, such as a 40 % increase in hospitalization for heart failure [73] and an increase in all-cause mortality (meta-analysis described in [74]).
It has been suggested that there could be heterogeneity in results due to the source of vitamin E; natural sources include multiple isoforms while synthetic sources are typically just α-tocopherol. As stated above, food sources contain mostly γ-tocopherol. This is important given the emerging evidence that γ-tocopherol may actually be a more powerful antioxidant than α-tocopherol (reviewed in [75]), and α-tocopherol may displace γ-tocopherol in vivo, suggesting that too much α-tocopherol inhibits the actions of γ-tocopherol [76]. In addition, α-tocopherol has even been observed under some circumstances to act as a prooxidant towards human LDL [77]. Overall, vitamin E supplementation may be beneficial in certain populations, but the potential for harm from chronic high exposure warrants caution.
β-Carotene (part of the vitamin A family) has also been proposed as an important plant-derived antioxidant and studied for a potential protective effect in CVD. Unfortunately, randomized controlled trials have found no benefit in either healthy [78] or at-risk women [68]. The β-carotene arm of the Women’s Health Study [78] was prematurely terminated due to both the lack of effect in a companion long-term study (12 years) of men [79] and the observed increase in risk among patients at high risk for lung cancer [80, 81]. High vitamin A levels from dietary intake did not correlate with any change in death from CHD in the Iowa Women’s Health Study [71]. Combined use of these and other antioxidants has also not proven beneficial [82, 83] and has even been observed to counteract the blood lipid-lowering effects of statin therapy [82].
Folic Acid and Other B Vitamins
Homocysteine is a cysteine homologue that serves as an intermediate in the biosynthesis of cysteine from methionine. Numerous observational studies have correlated high plasma homocysteine levels with CVD risk, including postmenopausal women with no previous history of CVD [84]. Of note, a 2002 meta-analysis suggested that a lower homocysteine level was more strongly associated with decreased CHD risk in women compared to men [85]. Plasma homocysteine levels have been shown to increase after menopause [86] and can be lowered by HRT [87, 88]. Supplementation with certain B vitamins, including folic acid (B9), B6, and B12, can significantly lower homocysteine levels by catalyzing the synthesis of cysteine from homocysteine. Therefore, numerous studies have tested whether B vitamin supplements can effectively decrease CVD.
In 2006, two randomized, placebo-controlled, double-blind studies were published. The HOPE-2 study [89] treated patients with existing vascular disease or diabetes with a combination of folic acid, B6, and B12 for 5 years, while the Norwegian Vitamin (NORVIT) trial [90] treated recent acute MI survivors with the same combination, just folic acid plus B12 or B6 alone for an average of 40 months. While both studies successfully lowered homocysteine levels in the folate groups, neither showed any benefit to recurrent CVD. In fact, the NORVIT results suggested a 22 % increase in cumulative CVD risk and a 30 % increase in nonfatal myocardial infarctions (MIs) with the triple combination therapy. In 2008, the Women’s Antioxidant and Folic Acid Cardiovascular Study (WAFACS), an expansion of the WACS trial, published the results of an ongoing trial in diagnosed or high CVD risk American women health professionals [91]. The women in this randomized controlled study also received a similar triple combination therapy and were monitored for CVD morbidity and mortality for over 7 years. The results confirmed previous studies: there was no difference in the combined endpoint from treatment with B vitamins, although homocysteine levels were significantly lowered. No harmful effects were noted in this study. Compared to secondary prevention studies, there is a relative lack of randomized controlled trials that consider B vitamins in the primary prevention of CVD in healthy women. In 1998, the prospective NHS did suggest that high folate and B6 intake led to an RR of 0.55 compared to women with the lowest intake levels of these vitamins [20]. However, the primary source of folate and B6 was from multi-vitamins, complicating analysis of this study. It is possible that elevated homocysteine levels are simply an indication of CVD but are not a direct cause, thereby explaining why interventions with B vitamins are not effective in preventing CVD.
Dietary Factors with Inconclusive Effects
Vitamin D
Vitamin D insufficiency has been hypothesized to contribute to the development of CVD [92]. Indeed, epidemiological evidence suggests that low levels of circulating vitamin D are associated with an up to 80 % increase in incident CVD [93]. Over 1/3 of otherwise healthy American young adults and even higher numbers of Europeans may have insufficient levels of vitamin D [94]. More than 60 % of postmenopausal women with osteoporosis may be vitamin D deficient [95]. Vitamin D has widespread effects throughout the body but is generally tied to calcium absorption. Vitamin D receptors are expressed both in cardiomyocytes and in blood vessels. In the context of CVD, vitamin D is speculated to protect from valvular and arterial calcification.
Several women-specific trials have studied the relation of vitamin D to CVD. In the Iowa Women’s Health Study, dietary questionnaires that included questions about calcium and vitamin D food sources and supplements were administered to 34,486 postmenopausal women at baseline; cardiovascular death was assessed within the 8-year study period [96]. While high calcium intake was associated with a 30–35 % reduction in CVD death, vitamin D proved ineffective in this large-scale, long-term study. However, the analysis was based on one questionnaire given at the onset, it only looked at death rather than overall CVD incidence, and it did not consider combined effects of calcium and vitamin D. The calcium/vitamin D supplementation arm of the WHI was a randomized, controlled trial that looked at CVD as a secondary outcome in 7 years of follow-up after daily dosing with 400 IU of vitamin D along with calcium [97]. Unfortunately, treatment had no effect on CVD. This study was complicated by background use among some patients of calcium supplements, poor adherence, and HRT, which has been documented to increase CHD incidence [50]. It is also possible that the dose used was inadequate, as optimal vitamin D intakes are still a contested issue. Of course, it is also conceivable that calcium/vitamin D is unrelated to the development of CVD. One other small, short-term trial involving women found no effect of high doses (2,500 IU/day) of vitamin D on endothelial function or arterial stiffness. The ongoing VITAL trial will test high doses of vitamin D (2,000 IU/day) in the primary prevention of cancer and CVD in healthy populations over the course of 5 years [98]. 20,000 middle-aged men and women are currently being recruited. This extensive trial will hopefully yield more insight as to the benefits, if any, of vitamin D supplementation towards CVD prevention.
Resveratrol
In 1992, Renaud described “The French Paradox,” which observes that, despite a diet relatively high in saturated fat, the French experience a very low mortality rate from CHD [99]. Alcohol consumption was the only dietary factor that could counteract the otherwise positive association of saturated fat with CHD mortality. An in vitro study, which demonstrated that the phenolic component of red wine in the absence of ethanol protected against LDL oxidation at 10 μM concentrations, quickly followed in 1993 and demonstrated that this fraction performed significantly better than α-tocopherol [100]. Resveratrol may also inhibit platelet aggregation and eicosanoid synthesis [101]. In vivo, resveratrol has been shown to prevent reperfusion-induced arrhythmias and mortality from ischemia–reperfusion injury in rats [102]. Micromolar plasma concentrations of polyphenols can be achieved in humans by ~2 glasses of red wine per day, suggesting that the in vitro effects may be clinically relevant [103]. In further support of the contribution of resveratrol to the French Paradox, resveratrol administration to mice on a high fat diet extended lifespan [104].
Clinical trials testing purified resveratrol in the prevention of CVD are scarce, but one ongoing trial (NCT01449110) is considering the effect of resveratrol on vascular health in postmenopausal women. Prospective studies in healthy women, including the NHS, have shown that moderate consumption of alcohol in the form of beer, wine, and liquor confers a 60 % protective advantage against coronary disease and also protects against stroke [15]. Very few of the women in the NHS qualified as heavy drinkers, therefore potential harmful effects at excessive levels could not be assessed. Moderate alcohol consumption also reduced risk in diabetic women, who are at high risk for CHD, by up to 52 % [105]. The role of resveratrol in these intriguing results would be significantly strengthened by randomized controlled trials in order to justify the growing popularity of resveratrol supplements.
α-Linolenic Acid
ALA is an intermediate chain n-3 fatty acid found in flaxseed, soybean, rapeseed, and canola oils as well as walnuts. It is an essential dietary fatty acid for humans. It is speculated that it has antiarrhythmic properties and thus can protect against SCD. After 18 years of follow-up, the Nurses’ Health Study reported a 38–40 % reduction in SCD when comparing the highest levels of ALA intake to the lowest [19]. They did not see such an association for nonfatal CHD. This is consistent with their earlier 10-year follow-up report [106]. As a prospective study, it is impossible to say whether ALA is directly responsible for the observed effect, but the results are intriguing. A companion study of men did not find a significant correlation of ALA with SCD, although there was association with other CHD indicators [107].
Small proportions of ALA (~8 %) can be converted to the marine long-chain n-3 fatty acids (primarily EPA but also DHA) in the liver, potentially linking these dietary factors (reviewed in [108]). Although this conversion rate is low, it has been reported to be 15 % higher in women than in men on controlled diets containing equivalent amounts of ALA and no EPA or DHA [109]. Estrogen signaling may account for this difference as oral estrogen administration to transsexual men increased DHA levels 42 % over control [109]. Given the potential sex difference in ALA effectiveness and metabolism but the dearth of randomized controlled trials, particularly for women, more investigation is certainly warranted.
Exercise
The Cardiovascular Effects of Chronic Endurance Exercise
Decades of research on the cardioprotective effects of exercise have led to consensus statements from both the AHA and the Council on Clinical Cardiology recommending regular physical activity for the prevention and modulation of CVD in men and women [24, 110]. Unfortunately, the current knowledge regarding these basic effects and how they modulate CVD risk and progression is mainly based on studies involving men only, despite data suggesting an important sexual dimorphism in this response. We therefore review the systemic and molecular mechanisms that are modulated by exercise with special consideration for those that are specific to women.
Adaptive Responses of the Cardiovascular System to Exercise in Males and Females
The cardiovascular system exhibits remarkable adaptive responses in response to exercise. Importantly, to efficiently circulate oxygenated blood to peripheral tissues, the strength and speed of cardiac contraction must be enhanced through the thickening of cardiac tissue (cardiac hypertrophy). In addition to cardiac morphologic adaptation, a complex feedback system involving the vasculature, nervous system, and metabolic processes in peripheral tissues modifies hemodynamics. Over time, the cardiovascular system becomes “toned” such that decreased sympathetic and increased parasympathetic activity reduces resting heart rate and blood pressure.
Although basic cardiovascular adaptations to exercise have been studied since the 1940s, the sexually dimorphic nature of cardiovascular responses to exercise has only recently been appreciated. Cardiac hypertrophy, for example, is observed in female rats that are swim-trained, whereas the hearts of male rats do not increase in size. As a result, females experience a greater contractile advantage with this form of exercise [111, 112]. Unlike with swimming, however, male rats that are treadmill-trained exhibit a morphological and functional cardiac advantage over females [113]. These data support the conclusion that the hearts of females and males respond differently to various forms of exercise.
Both sexes also exhibit distinct vascular adaptation in response to exercise, stemming from differential endothelial and autonomic activity. Interestingly, women exhibit enhanced basal parasympathetic tone compared to age-matched men, which is thought to account for much of the cardioprotection experienced by younger women, even in the absence of exercise [114, 115]. Recent data on sexually dimorphic responses to exercise emphasize a need for creating unique recommendations for men and women. Because these outcomes are best described for endurance training compared to resistance or strength training, we will focus on the protective and modulatory effects of aerobic exercise.
Exercise Reduces Risk and Progression of Cardiovascular Disease
It is well-established that engaging in regular aerobic exercise lowers the risk of developing CVD; the rate of many forms of CVD in physically active people is significantly lower than in those who are sedentary [110, 116, 117]. Correspondingly, women report exercise as the number one strategy for reducing the risk of developing CVD [9]. However, medical professionals are less likely to advise women than men to engage in exercise [118]. This dichotomy may be due to the relative lack of evidence-based studies in women: the most comprehensive report in recent years, the United States Surgeon General’s Report on Physical Activity and Health, considered women in only 2 % of the studies [119]. Additionally, from 1950 to 1995, of 43 clinical studies examining the relationship between exercise and CVD risk, only seven included women [120]. Interestingly, each of the small number of early studies involving women defined exercise as housework, employment-related tasks, or leisure time activities [121–124], a definition thought to be sufficient because about 82 % of physical activity could be attributed to housework [125]. Despite the conservative description of exercise, the notion that physical activity is cardioprotective in women is supported in current research.
The type, intensity, and duration of exercise can have a significant and unique impact on the degree of cardioprotection experienced by men and women [126]. Early studies hypothesized that intense exercise exerts a more dramatic benefit than moderate activity, albeit in men [127]. Because walking is a popular form of exercise among women [128], defining the unique cardiovascular effects of low or high intensity activity was necessary. A prospective study of middle-aged women demonstrated that low intensity exercise is at least as beneficial as vigorous exercise, suggesting that walking is a sufficient method for reducing risk; indeed, walking led to about a 40 % reduction in CVD over the 10-year study [129]. Other recent large studies demonstrated that as little as 2 h of activity per week can promote this benefit [125, 130, 131]. Interestingly, the pace or intensity of exercise in women did not affect risk of developing CVD, an effect that also held true for women who had other risk factors including diabetes and cigarette smoking [130]. The data strongly suggest that reasonable recommendations could be made for sedentary women, a population previously thought to be resistant to engaging in strenuous exercise programs like running [128]. Basic recommendations for women have therefore been updated to include mild to moderate exercise, such as walking 30 min a day, at least 5 days a week for the prevention of CVD [132].
In addition to modulating risk, exercise has been convincingly shown to slow the progression of existing CVD in both men and women [133–136]. Despite these data, less than 20 % of patients with cardiac events participate in cardiac rehabilitation programs, including those that involve exercise regimens [137]. Women are even less likely to participate in exercise rehabilitation after MI [138], likely a result of a lack of evidence-based studies; prior to 1990, meta-analyses on the modulation of CVD by exercise excluded women [139]. In light of dramatic differences in the manner in which men and women respond to CVD, these studies are required. Women with hypertension or aortic stenosis, for example, exhibit greater contractility, enhanced hypertrophy, and better prognoses compared to men [140, 141]. However, with ischemic attack, men fare better than women [142]. Interestingly, the few recent studies that included women did not include men, so identification of possible sex differences in response to specific forms of exercise has not been achieved. One small study demonstrated women participating in exercise rehabilitation programs experienced greater functional cardiac benefits to exercise than men with existing CVD [143]. However, the women considered had poor risk profiles (higher rates of diabetes and hypertension) compared to men and no distinction was made among the various forms of CVD. Thus, the type of pathologic insult appears to be important in terms of baseline sexual dimorphism and may dramatically affect how the patient responds to an exercise rehabilitation program. Because of a lack of detailed information on the effects of exercise on existing CVD, we focus on cardioprotective effects of exercise in individuals without CVD.
Protective Effects Induced by the Cardiovascular Responses to Exercise
Recent conflicting studies demonstrate effects of exercise ranging from significant positive modulation to no change on many systemic and molecular processes. Discrepancies are likely due to the diversity of types, durations, and intensities of exercise utilized, each of which has unique effects on the cardiovascular system [144]. However, a number of mechanisms have been proposed with convincing supporting data, many of which exhibit sexually dimorphic effects. Here, we review current clinical and basic science research supporting the notion that the cardiovascular systems of women and men respond differently to exercise and explore the mechanisms that are thought to modulate effects of exercise on the cardiovascular systems of women.
Although much of the cardiovascular benefit of exercise is attributable to responses of the autonomic nervous system and vasculature, it should be noted that many processes contribute to the cardiovascular benefit of exercise in both men and women. Sensitivity of various tissues to insulin, levels of serum lipids, and coagulative properties of blood can directly and indirectly influence cardiac health. In the broadest sense, physical activity also alters metabolism and, not surprisingly, decreases body weight and adiposity [145]. Decreased blood pressure [146, 147], reduced serum triglycerides, and increased HDL [148–150] are also consistently observed among individuals who engage in regular exercise programs. As a result, exercise essentially reduces all symptoms of metabolic syndrome [151], thereby indirectly improving cardiac processes associated with this syndrome. Many of these responses have been studied in men and women; results reveal a significant sexual dimorphism in basal function and alterations due to physical activity.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree