Eugene Hughes
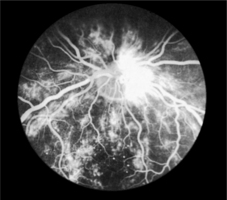
Fig. 9.1Fluorescein angiogram of the retina
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



9. Microvascular disease
CHAPTER 9. Microvascular disease
Diabetic retinopathy 196
Diabetic nephropathy 203
Diabetic neuropathy 206
Autonomic neuropathy 209
Erectile dysfunction 211
Conclusion 214
References 214
Diana is a 42-year-old married woman with type 1 diabetes. She has recently moved into the area and applies to join a new GP practice. She makes an appointment to see the GP as she has developed tingling and numbness in both her feet. She complains of a burning discomfort in her legs that is worse during the night and prevents her from sleeping.
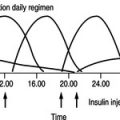
Diana has had diabetes for almost 20 years and is on a twice-daily regime of a fixed mixture of insulin (soluble and isophane). She says that her diabetes is satisfactorily controlled and she never has any problems with hypoglycaemia. In conversation, it is clear that she rarely monitors her blood glucose and has not attended a diabetic clinic for more than 8 years. Her previous GP did not run a diabetic clinic and she has not received any screening for diabetic complications during this period of time. Diana’s GP finds that her blood glucose is measured at 14.6 mmol/L and her long-term control has been suboptimal indicated by an HbA1c of 10.2%. Diana states that she has never smoked and drinks only three units of alcohol per week.
On examination, Diana has reduced sensation to both light touch and pin-prick testing to mid-calf level in both legs. Her blood pressure is noted to be elevated at 165/95 mmHg; both her urea and creatinine are also elevated at 15.8 mmol/L and 270 micromol/L, respectively. Diana is referred to the local eye-screening clinic and advanced background retinopathy is confirmed.
Diana, after living with diabetes for nearly 20 years, has now developed some of the complications of diabetes namely hypertension, retinopathy, nephropathy and neuropathy. Diana’s hypertension would be managed according to the recommendations in Chapter 8.
INTRODCUTION
Microvascular disease occurs in people with both type 1 diabetes and type 2 diabetes. The hallmark of diabetic microvascular disease is microangiopathy (small vessel disease). This is the underlying abnormality that leads to retinopathy, nephropathy and neuropathy.
In microangiopathy, thickening of the capillary basement membrane leads to increased capillary permeability at an early stage. The development of microangiopathy is intricately linked with hyperglycaemia, and the duration of hyperglycaemia is an important determinant of microvascular disease. Individuals with type 1 diabetes can survive for many years without showing any signs of microvascular disease, particularly if they have optimal glycaemic control. Those with type 2 diabetes tend to be older at presentation and, even though they commonly have features of microvascular disease, it is ultimately macrovascular disease that leads to their untimely death. The exact biochemical mechanism of microvascular disease is not within the remit of this chapter; however, the central mechanism appears to relate to the sorbitol pathway. Hyperglycaemia leads to increased accumulation of sorbitol which does not easily cross cell membranes. The biochemical consequences of this directly affect the proteins in the vessel walls (Williams & Pickup 2004).
Diabetic microvascular disease is associated with the development of complications in people with both type 1 and type 2 diabetes. This section discusses the following microvascular complications:
▪ diabetic retinopathy
▪ diabetic nephropathy
▪ diabetic neuropathy
▪ autonomic neuropathy
▪ erectile dysfunction (impotence).
For each of these complications, the prevalence, pathological features, screening, protocols for early referral and – where relevant – the implications of the national service framework for diabetes and the general medical services contract for primary care will be considered (Kenny 2004).
DIABETIC RETINOPATHY
THE FACTS
▪ Diabetes is the most common cause of blindness in people aged 30–69 years and the most common cause of blindness in the working population of developed countries (Cormack et al 2001, Icks et al 1997).
▪ 2% of the UK diabetes population are registered blind.
▪ The condition is treatable by laser photocoagulation.
FACTORS INFLUENCING THE DEVELOPMENT OF RETINOPATHY
▪ The duration of diabetes.
▪ The presence of hypertension.
▪ The development of chronic renal failure.
▪ The level of glycaemic control.
▪ Lifestyle factors including smoking and alcohol.
▪ Race as it is more common in certain ethnic groups.
It is clear from Diana’s case study that she meets three of these: diabetes for almost 20 years; hypertension and poor glycaemic control.
CLASSIFICATION
Diabetic retinopathy is graded into four main categories, depending on the extent of the disease seen on fundal examination.
BACKGROUND RETINOPATHY
As capillaries close, the retina becomes underperfused with blood. Surrounding capillaries dilate in response to this and microaneurysms form. These tiny pin-head red dots are the first lesions to be seen in developing retinopathy. Dilated capillaries are usually leaky and proteinaceous material escapes forming creamy yellow exudates (hard exudates) on the retinal surface. Large blot haemorrhages tend to form at the interface of the well-perfused and ischaemic areas of the retina (Fig. 9.1).
ADVANCED BACKGROUND RETINOPATHY
If extensive leakage from capillaries occurs around the macula, macular oedema develops. As the macula is involved with central vision, oedema of this area can markedly impair vision. The person will usually complain of blurring of vision, particularly central vision, which is used when reading. As more and more capillaries become occluded, large areas of the retina become ischaemic and cotton wool spots (or soft exudates) develop at sites of retinal microinfarction. The veins of the retina also begin to dilate. The veins form loops or show beading and reduplication. The presence of cotton wool spots and venous changes suggest that new vessel formation is imminent. Approximately one-third of people will develop new blood vessel formation within the next 2 years and progress to proliferative retinopathy. People with advanced background retinopathy should therefore be referred to an ophthalmology clinic. Diana was found to have this when she underwent screening.
PROLIFERATIVE RETINOPATHY
Ischaemic areas of the retina subsequently give rise to new blood vessel formation. These new vessels usually arise from veins in the retinal periphery or on the optic disc. At first they lie on the surface of the retina but eventually they grow forwards, attaching themselves to the posterior surface of the vitreous, which lies immediately in front of the retinal surface. As the vitreous detaches, it pulls on the new vessels, causing them to rupture and haemorrhage. Haemorrhaging into the vitreous causes sudden loss of vision as the blood prevents light reaching the retina behind. Vitreous haemorrhages usually clear gradually over a period of days or weeks with vision slowly recovering. Urgent referral to an ophthalmologist is recommended.
ADVANCED RETINOPATHY
Repeated vitreous haemorrhages stimulate fibrous tissue proliferation. Fibrous strands arising in relation to new vessels begin to contract and as this process gradually progresses, the retina become detached with resulting loss of vision.
COMPONENTS OF RETINOPATHY
▪ Small, round microaneurysms: dots.
▪ Medium-sized haemorrhages: blots.
▪ Hard exudates: irregular yellow lipid deposits.
▪ Cotton wool spots: white indistinct ischaemic areas.
▪ New vessels: fine tangled loops of vessels.
Background retinopathy
▪ Microaneurysms.
▪ Dot and blot haemorrhages.
▪ Hard exudates.
▪ Cotton wool spots.
Preproliferative retinopathy
▪ All of the above plus venous beading and looping and larger haemorrhages.
Proliferative retinopathy
▪ New vessels at the disc or in the periphery.
▪ Increased fibrosis.
▪ Retinal detachment.
Maculopathy
▪ Ischaemia or oedema of the macula area.
SCREENING AND PREVENTION
Prevention of diabetic blindness is possible in most people as long as treatment is initiated early. As the initial features of retinopathy are symptomless, screening is worthwhile and effective at detecting this and should be started from the age of 12 years (Scottish Intercollegiate Guidelines Network (SIGN) 55 2001). In Diana’s case, she had not had any eye screening for at least 8 years and it was fortunate that only background retinopathy was found.
Impaired vision is an early feature of maculopathy and can develop before any retinal changes are evident on ophthalmoscopic examination. A fall in visual acuity is therefore the first sign of serious maculopathy developing. By contrast, people with proliferative retinopathy will be unaware of any eye problem until a vitreous haemorrhage occurs, which causes a sudden loss of vision. By this stage retinopathy will be advanced and difficult to treat effectively. It is evident therefore that any screening programme must include both the routine measurement of visual acuity and fundoscopy or retinal photography (SIGN 2001).
VISUAL ACUITY
Distance vision is measured with a well illuminated Snellen chart at 6 metres. Each eye is tested separately, while the other eye is covered with a card. Visual acuity is quoted as the smallest letters that can be read. Thus, if a person can only read at 6 metres letters that should normally be read at 24 metres, visual acuity is recorded as 6/24. Normal vision is 6/6 but those with long sight and young people can often manage 6/5 or 6/4. Vision deteriorates with age and 6/9 vision is not necessarily abnormal in the elderly. If no letters can be read, the ability to count fingers (CF), identify hand movements (HM) or perceive light (PL), should be tested and recorded. Vision should be tested with the person wearing glasses if these are normally used for distance. If vision is impaired, the test should be repeated viewing the chart through a hole punched in a card (a pinhole). This partially corrects refractive errors. The best acuity measurement for each eye should be recorded.
Tests of reading ability using cards with varying sizes of script test central vision and are therefore particularly sensitive to macula changes.
FUNDAL EXAMINATION
Fundoscopy should be carried out only by doctors with training and experience of looking at the eyes of people with diabetes. For this reason, most GPs prefer to send individuals with diabetes to an ophthalmology clinic for retinal examination. In some areas of the UK, local opticians have been trained in assessing diabetic fundi and are used in screening programmes.
To obtain full and clear views of the retina, it is necessary for the pupils to be dilated by using mydriatic drops. Tropicamide (0.5% or 1%) one drop in each eye with phenylephrine hydrochloride 2.5% one drop in each eye (added after to maximise dilatation) are the most suitable agents because they act rapidly and wear off within 6 hours. There is little to be gained from reversing the dilatation using pilocarpine after the consultation. Drops are usually initiated by nursing staff, who should first check that the person does not suffer from glaucoma. The person should be advised previously that an eye examination will happen and warned that his or her vision may remain blurred and/or sensitive to light for up to 6 hours after the examination. Individuals might need to wear dark glasses and driving during this period is not recommended.
The examination should take place in a suitably darkened room using an ophthalmoscope with a fully charged battery providing adequate illumination.
In the UK a national retinal screening program is underway using non-mydriatic digital retinal cameras. Administrative call and recall procedures link diabetes registers to the precess where trained screeners and graders offer a quality assured service.
RETINAL PHOTOGRAPHY
Retinal screening by ophthalmoscopy should be considered the bare minimum standard of screening. Retinal photography as part of a local or regional scheme should be accessible to most people with diabetes. The National Service Frameworks for Diabetes recommended that retinal screening programmes be put into place throughout the UK by 2006. Leese et al (2005) found that robust screening resulted in more appropriate referrals to ophthalmologists. Digital retinopathy with assessment and a grading is likely to become the gold standard in most areas. This has the advantage of being able to store and compare images with successive assessments. Digital photographs can also be used for telemedicine in remote and rural areas where there is robust quality assurance it is practical and advantageous (Schneider et al 2005). Criteria for referral to ophthalmologist are detailed in Box 9.1.
Box 9.1
Criteria for referral to an ophthalmologist
Routine referral
▪ Cataract
▪ Non-proliferative retinopathy
Early referral
▪ Preproliferative changes
▪ Retinal haemorrhages and perimacular hard excudates
▪ Decreasing visual acuity (because this might indicate maculopathy)
Urgent referral
▪ New vessels
▪ Rubeosis iridis
Immediate referral
▪ Vitreous haemorrhage
▪ Neovascular glaucoma
▪ Advanced diabetic eye disease including retinal detachment
GENERAL MEASURES FOR PREVENTION AND TREATMENT
As for all microvascular and macrovascular complications, improving clinical and lifestyle factors will help prevent the onset and deterioration of diabetes complications (Diabetes Control and Complications Trial (DCCT) 1993):
▪ tight glycaemic control
▪ tight blood pressure control
▪ tight lipid control
▪ smoking cessation.
SPECIFIC TREATMENT
Photocoagulation
Laser photocoagulation is particularly effective in preventing visual loss due to new vessel formation. To be effective it must be given early. In 90% of people the new vessels disappear or become insignificant. In ischaemic maculopathy, treatment is less effective. However, in clinically significant macula oedema, laser treatment has been show to be beneficial (SIGN 2001). For proliferative retinopathy, photocoagulation is applied to a larger area of the retina (pan retinal photocoagulation). This can involve between 1500 and 7000 separate burns and can be performed over several sessions of outpatient treatment, following the application of topical local anaesthetic drops. Some people experience mild discomfort with this procedure. If severe discomfort is experienced, retrobulbar local anaesthetic can be given. The underlying principle of photocoagulation is that it halts deterioration; it might not lead to improved visual acuity.