Prevention and Adjuvant Therapy in Breast Cancer
Mayo Clinic, Jacksonville, FL, USA
• What are the best strategies for breast cancer prevention in postmenopausal women?
There are essentially two approaches to optimize breast cancer prevention in postmenopausal women. The first strategy consists of lifestyle modifications, such as exercising regularly, decreasing alcohol use, and minimizing exposure to combined estrogen and progesterone exogenous hormones. Regarding exercise, at least 60 cohort and case studies have examined the relationship between exercise and primary breast cancer prevention. Although the evidence has not all been consistent, most findings suggest a 15% to 20% risk reduction for women who exercise regularly compared to those who remain sedentary. The second strategy consists of chemoprevention. Currently, two selective estrogen receptor modulators (SERMS), tamoxifen and raloxifene, have been approved by the US Food and Drug Administration (FDA) for breast cancer prevention. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 trial, tamoxifen significantly deceased the number of invasive breast cancers by 49% (P < 0.001) compared to placebo. Similar risk reductions were found with raloxifene. However, both raloxifene and tamoxifen have increased risk of venous thromboembolism, and tamoxifen is associated with an increased risk of endometrial cancer. Given the toxicity profiles of these two drugs, and their perhaps lower-than-anticipated patient acceptance, they have failed to gain full adoption for primary breast cancer prevention.
Aromatase inhibitors (AIs) do suppress estrogen levels in postmenopausal women and are part of standard therapy for patients with early- or advanced-stage estrogen and/or progesterone receptor–positive breast cancer. Moreover, both nonsteroidal and steroidal AIs have been demonstrated to reduce contralateral primary breast cancers compared to tamoxifen or placebo in patients with early breast cancer, with an arguably better tolerability profile. These observations led to the MAP.3 study, a phase III, randomized, double-blind trial of exemestane (a steroidal AI) versus placebo for primary breast cancer prevention in postmenopausal women. A total of 4560 postmenopausal women with a median Gail risk score of 2.3% received exemestane at 25 mg daily for 5 years. After a median follow-up of 35 months, exemestane significantly reduced the relative incidence of invasive breast cancers by 65% in postmenopausal women compared to placebo, with an annual incidence of invasive breast cancer of 0.55% with placebo compared to 0.19% in the exemestane group (hazard ratio (HR): 0.35; P = 0.002). Additionally, exemestane reduced the risk of known breast cancer precursor lesions such as ductal carcinoma in situ (DCIS), lobular carcinoma in situ (LCIS), atypical ductal hyperplasia, and atypical lobular hyperplasia. In terms of tolerability, there were no significant differences between the two groups in terms of skeletal fractures, cardiovascular deaths, other cancers, treatment-related deaths, or quality of life. Although not currently FDA approved for primary breast cancer prevention, exemestane or anastrozole administered for 5 years is a reasonable option for primary breast cancer prevention in postmenopausal women. Ongoing trials will further improve our understanding of the long-term efficacy and toxicity of AIs, as well as the optimal duration of therapy.
• What is the role of CYP2D6 in the efficacy and tolerability of tamoxifen in the chemoprevention setting?
There are currently two SERMs that are approved by the FDA for the chemoprevention of breast cancer, tamoxifen and raloxifene. Tamoxifen, a weak anti-estrogen, is metabolized in vivo to potent anti-estrogens, 4-hydroxy tamoxifen and 4-hydroxy N-desmethyl tamoxifen (also known as endoxifen, felt to be the most abundant and active metabolite of tamoxifen). The metabolism of tamoxifen is mediated by several of the cytochrome P450 enzymes, including the CYP2D6-mediated oxidation of endoxifen.
Controversy exists regarding the association between CYP2D6 phenotype and the effectiveness of tamoxifen in the adjuvant and metastatic settings for invasive breast cancer. Depending on race, it has been estimated that 50% of women are thought to be extensive metabolizers (EMs), 43% are intermediate metabolizers (IMs) with reduced activity of CYP2D6, and 7% are poor metabolizers (PMs) with essentially negligible CYP2D6 enzyme activity. These genotypic variations of CYP2D6 have been showed to affect endoxifen concentration. Both IMs and PMs are likely to have decreased concentrations of endoxifen, which has been hypothesized to reduce the effectiveness of tamoxifen.
Numerous retrospective studies have shown conflicting results (i.e., both positive and negative associations of the CYP2D6 genotype and inhibition with tamoxifen efficacy). This heterogeneity of data was also seen in three large adjuvant clinical trials. Both the Breast International Group (BIG) I-98 clinical trial and the Arimidex, Tamoxifen, Alone or in Combination (ATAC) trial found no statistically significant associations between the CYP2D6 genotype and breast cancer recurrence in tamoxifen-treated postmenopausal women. These two clinical trials are in contrast to the Austrian Breast and Colorectal Cancer Study Group (ABCSG) 8 clinical trial, in which PMs of CYP2D6 treated with 5 years of tamoxifen were shown to have a statistically significant increased risk of breast cancer recurrence compared to EMs. However, for patients who were randomized to 2 years of tamoxifen followed by 3 years of anastrozole, the CYP2D6 genotype was not associated with increased disease recurrence. However, there was a trend toward nonsignificant higher odds of a disease event among PMs of CYP2D6 relative to EMs in the first 2 years of tamoxifen similar to those who received tamoxifen alone, but no such trend was seen during the 3 years of anastrozole treatment, suggesting that the use of AIs after tamoxifen negates the trend toward disease recurrence. Overall, the data regarding the role of tamoxifen metabolism and clinical outcomes are inconsistent and remain controversial. There are currently prospective studies designed to test whether measured activity of CYP2D6 and other metabolizing enzymes significantly affects clinical adjuvant outcome to warrant routine testing.
Studies in the primary prevention setting are also worthy of mention. In a subgroup analysis of a small number of patients in the Italian Tamoxifen Prevention Trial, PMs of CYP2D6, of which there were only eight patients, had a higher risk of developing breast cancer compared to women who were EMs or IMs. This result suggested that a pharmacogenetic work-up for CYP2D6 may help to tailor tamoxifen therapy for breast cancer prevention to those who are most likely to benefit. Indeed, Irvin and his colleagues (2011) did demonstrate that it was feasible to have genotype-driven dosing of tamoxifen. Doubling the dose of tamoxifen did increase the endoxifen concentration for IMs and PMs. Interestingly, only with IMs did the endoxifen concentration reach the level found in EMs. However, in a nested case–control study using data from the National Surgical Adjuvant Breast and Bowel Project (NSABP) P1 and P2 prevention clinical trials, Goetz and colleagues (2011) sought to analyze the association between CYP2D6 genotype, CYP2D6 inhibitor use, and the combination of both with breast cancer events in women who received tamoxifen or raloxifene for the prevention of breast cancer. No association was found between the CYP2D6 genotype and the development of breast cancer in either the tamoxifen or raloxifene arms. Additionally, no association between the odds of developing breast cancer and the use of either a potent or weak CYP2D6 inhibitor with tamoxifen or raloxifene was found.
At this time CYP2D6 genotyping is not considered part of clinical standard of care for decisions related to the use of tamoxifen.
• What are some of the genetic assays used to predict the risk of breast cancer recurrence, and how do they differ?
Since the early 2000s, genetic assays have emerged as useful tools for assessing the risk of recurrence in patients with early-stage breast cancer. Several commercially available multiple-gene assays have been validated in node-negative breast cancer patients, including Oncotype DX™ (Genomic Health), MammaPrint™ (Agendia), Mammostrat™ (Clarient), and IHC4™. Both MammaPrint and Oncotype DX are based on gene expression profiling, whereas Mammostrat and IHC4 are based on immunohistochemistry (IHC) or protein expression profiling (see Table 81.1).
Table 81.1 Comparison of Oncotype DX™, MammaPrint™, Mammostrat™, and IHC4™.
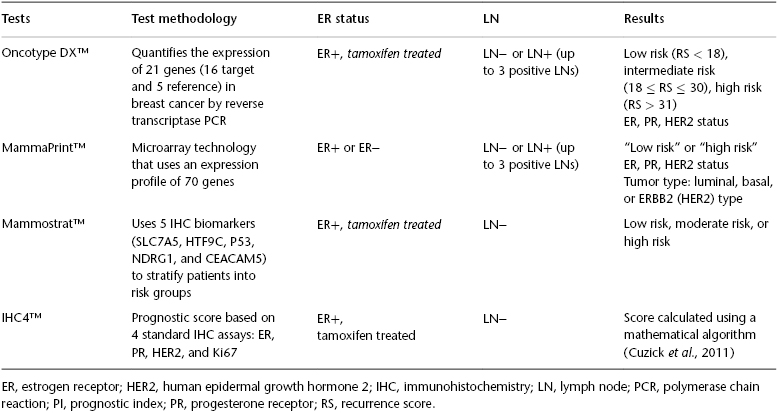
MammaPrint was developed in the Netherlands as a tool to help clinicians determine which patients with early breast cancer will develop metastases after curative surgery and radiotherapy (without systemic therapy). Using a 70-gene microarray, it stratifies women with hormone receptor–positive or –negative, lymph node–negative or –positive breast cancer to either a “low risk” or “high risk” of distant recurrence. Those women with low risk have a ∼10% risk of developing distant metastases in the next 10 years without any adjuvant hormonal or chemotherapy. Those who are “high risk” have a 30% risk of distant recurrence and are thought to benefit from both neo-adjuvant and adjuvant chemotherapy. MammaPrint is currently the only FDA-approved prognostic and predictive assay, although its predictive ability for benefit to standard therapies needs to be better defined.
Mammostrat, as opposed to MammaPrint or Oncotype DX, stratifies patients into low-, moderate-, and high-risk groups by measuring the protein-level expression of five biomarkers (SLC7A5, HTF9C, P53, NDRG1, and CEACAM5) in tumor tissue. It provides a score that predicts the 10-year risk of distant recurrence for ER+, node-negative breast cancer after 5 years of adjuvant endocrine therapy. In validation studies using archived samples from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 and B-20 trials, it was found that women with “low risk” had a 7.6% chance of distant recurrence in 10 years, while those with “moderate risk” and “high risk” had a 16.3% and 20.9% chance of distant recurrence in 10 years, respectively. This platform has also been recently reported to be a good prognostic classifier in the adjuvant hormonal setting of breast cancer, in the context of the Tamoxifen Exemestane Adjuvant Multinational (TEAM) study.
IHC4 is a protein expression-profiling prognostic tool based on quantitative values of four standard laboratory assays (estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), and Ki67). IHC4 is combined with clinicopathologic parameters of tumor grade, size, nodal burden, and treatment with an AI or tamoxifen (IHC4 + clinical score (IHC4 +C)). The IHC4 score gives prediction of distant recurrence at 9 years for postmenopausal women with node-negative, hormone receptor–positive cancer treated with 5 years of adjuvant endocrine therapy. The IHC4 +C was found to have comparable prognostic information similar to that of Oncotype DX using the TransATAC data set and was also validated in an independent data set. A major advantage of IHC4 +C is its cost-effectiveness, as it is considerably less expensive than gene expression profiling tools. Another advantage is that it uses existing laboratory assays and in theory could be performed in the majority of clinical laboratories. However, lack of standardization of these assays may make implementation of this prognostic tool difficult.
The best-known and most utilized test is Oncotype DX. Oncotype DX is a 21-gene assay that uses a panel of 16 cancer-related genes and 5 reference genes to predict the likelihood of developing distant recurrence in estrogen receptor (ER)-positive, early-stage breast cancer. This recurrence score (RS) predicts a 10-year risk of distant recurrence after 5 years of adjuvant hormonal therapy. Ranging from 0 to 100, the RS can be subdivided into three risk categories: low (<18), intermediate (18–30), and high (>31) scores. This 21-gene assay has been shown to quantify the likelihood of breast cancer recurrence in several validation studies. In a validation study using paraffin-embedded tissue blocks from the NSABP B-14 tamoxifen-treated cohort, Kaplan–Meier estimates of the rates of distant recurrence at 10 years in the low-risk, intermediate-risk, and high-risk categories were 6.8%, 14.3%, and 30.5%, respectively. There are many other panels reported to play a role in determination of prognosis, most recently expanded with the availability of the PAM50 assay. Further studies will be required to optimize the utilization of all of these panels in clinical practice.
• Is molecular profiling needed for every patient diagnosis with invasive breast cancer?
No, not every diagnosis. For many years, hormonal therapy with tamoxifen was the gold standard for patients with node-negative, ER-positive breast cancer. Then, in 1997, the NSABP-20 trial showed a significant benefit in adding chemotherapy to tamoxifen, although the absolute benefit was relatively modest. As a result of this clinical trial and others, many women with ER-positive, node-negative breast cancer receive combination chemotherapy and hormonal therapies, with the understanding that not all benefit from that approach.
A series of pivotal trials incorporating genomic profiles will help figure out who should routinely undergo the available tests, to determine whether there is significant benefit to adding chemotherapy for relevant subsets for which the retrospective studies done are not clear. The Microarray in Node Negative Disease May Avoid Chemotherapy (MINDACT) trial is an ongoing prospective, randomized clinical trial that will compare risk assessment using MammaPrint profile with risk assessment using the clinicopathological criteria of Adjuvant! Online. The results of the MINDACT trial should be available in 2014. The rationale for this study is strong. MammaPrint is currently the only FDA-approved multigene prognostic and predictive assay for early-stage breast cancers irrespective of hormone receptor or nodal status. MammaPrint was found to provide prognostic information beyond what was determined by the patient’s age, tumor grade, tumor size, and ER status in a population of node-negative patients who did not receive adjuvant hormonal therapy or chemotherapy. It also performed better than outcome assessments derived from Adjuvant! Online. Indeed, there was 28–35% discordance between MammaPrint and Adjuvant! Online.
The Trial Assigning Individualized Options for Therapy (TAILORx) Trial is the second prospective trial eagerly awaiting analyses. In this trial, patients with an RS of 25 or higher will receive chemotherapy plus hormonal therapy, and patients will an RS lower than 11 will receive only hormonal therapy. Those with an RS between 11 and 25 will be randomly assigned to receive chemotherapy and hormonal therapy or hormonal therapy alone. It is noted that the intermediate group of TAILORx (women with an RS between 11 and 25) is different from the definition of “intermediate” in commercially available tests, where the score is between 18 and 31. This has prompted some physicians to offer chemotherapy to patients with a recurrence score greater than 26, to coincide with the study. The TAILORx study completed accrual, with data expected in 2017.
According to 2007 American Society of Clinical Oncology (ASCO) guidelines, in newly diagnosed patients with node-negative, estrogen receptor–positive breast cancer, the Oncotype DX assay can be used to predict the risk of recurrence in patients treated with tamoxifen. Oncotype DX may be used to identify patients who are likely to benefit from tamoxifen therapy alone and may not require adjuvant chemotherapy. These 2007 guidelines were limited only to women treated with tamoxifen; however, a later study showed that the 21-gene recurrence score can also be applied to postmenopausal women treated with anastrozole as well. Combining the ASCO guidelines with National Comprehensive Cancer (NCCN) guidelines, molecular profiling based on tumor size may be the most cost-effective option. For node-negative or micrometastatic (≤2 mm axillary node metastases) tumors that are less than 0.5 cm, molecular profiling is not needed as adjuvant chemotherapy is usually not recommended. For patients with node-negative tumors between 0.6 and 1.0 cm, molecular profiling can help guide therapy. For tumors larger than 1.0 cm, adjuvant chemotherapy is usually recommended. Adjuvant chemotherapy is also recommended for lymph node–positive breast cancer regardless of tumor size, although there is some evidence that gene profiling expression can also be used for women with three or fewer positive lymph nodes. The results of the MINDACT and TAILORx trials will further help clarify the role of molecular profiling for many patients.
• How accurate is HER2 testing in breast cancer?






