Screening, Staging, and Stage I Non-Small-Cell Lung Cancer
Cancer Institute of Florida, Orlando, FL, USA
Over the past 4 decades, a large amount of research has been performed to evaluate if conventional radiography or CT could be effective screening tests for lung cancer. Previous screening studies with chest radiographs and/or sputum cytology have failed to show any mortality reduction from lung cancer from those tests. The Prostate, Lung, Colorectal, and Ovarian cancer screening trial compared screening with chest radiograph to observation and detected no mortality reduction from lung cancer in the screened population. These earlier randomized controlled trials (RCTs) showed that chest radiographs detected slightly more lung cancers and more stage I tumors, but failed to demonstrate decreased mortality from lung cancer, or a stage shift, defined as follows: increase detection of early cancer and decreased incidence of late stages.
The advent of low-dose (radiation) computed tomography (LDCT) imaging created renewed interest in screening. In the past 10 to 15 years, there have been a large number of clinical trials evaluating the role of LDCT screening for asymptomatic lung cancer. Early studies demonstrated that when a chest X-ray was obtained within 30 days of an LDCT scan, the chest X-ray missed 70% to 80% of the LDCT-detected lung cancers. Many of these early lung cancers detected on these trials were curable by surgical resection. These nonrandomized trials, however, have many inherent limitations that hamper our ability to draw definitive conclusions. These limitations include the potential for overdiagnosis bias, lead-time bias, and disease-type bias.
- Overdiagnosis bias occurs when a screening test identifies disease that never would have affected the patient’s life in the absence of screening. This type of bias might occur if screening identifies indolent lesions that would have never caused clinical disease.
- Lead-time bias occurs when screening results in earlier recognition of disease, but does not change the patient’s eventual lifespan, creating the illusion that the patient’s survival time with the disease is longer.
- Disease-type bias arises from the observation that any screening test that is applied intermittently is more likely to detect indolent tumors than aggressive, fast-growing tumors that would result in clinical symptoms.
Randomized Controlled Trials
Three randomized studies provided evidence on the effect of LDCT screening on lung cancer mortality, of which the National Lung Screening Trial (NLST) was the most informative.
The NLST
The NLST included 53,454 persons at high risk for lung cancer. Participants were randomly assigned to undergo three annual screenings with either low-dose CT or single-view postero-anterior chest radiography. These three annual rounds of screening (baseline and 1 and 2 years later) with LDCT resulted in a 20% relative decrease in deaths from lung cancer versus chest radiographs over a median of 6.5 years of follow-up (P = 0.004). In absolute terms, the chance of dying from lung cancer was 0.33% less over the study period in the LDCT group (87 avoided deaths over 26,722 screened participants).
It is important to know that the rate of positive screening tests was 24.2% with LDCT and 6.9% with radiography over all three rounds. A total of 96.4% of the positive screening results in the LDCT group and 94.5% in the radiography group were false-positive results. This emphasizes the need for careful and structured evaluation protocols for patients with positive screening findings to avoid unnecessary interventions.
Two other considerably smaller ongoing studies, DANTE and DLCST, each compared five annual rounds of LDCT screening to usual care; after a median of 34 and 58 months of follow-up, respectively, no statistically significant difference in lung cancer mortality was observed in either study (DANTE: relative risk (RR), 0.97; 95% CI, 0.71–1.32; P = .84; DLCST: RR, 1.15; 95% CI, 0.83–1.61; P = .43).
Since the publication of the NSLT results, many organizations, including the American Thoracic Society, American Society of Clinical Oncology, National Comprehensive Cancer Network, and American Cancer Society, have endorsed LDCT screening for high-risk populations, as defined on the NSLT. A substantial amount of data on LDCT screening should be reported in the near future, including numerous planned analyses of the NLST data both by its investigators and by the Cancer Intervention and Surveillance Modeling Network (CISNET) investigators. The ongoing RCTs in Europe will also be reporting estimates of both the magnitude of LDCT’s mortality benefit and the extent of its harms soon. These data may help inform some of the important questions that still linger regarding LDCT screening.
False-Positive Results
LDCT identifies both cancerous and benign nodules; the latter are often called “false positives.” Based on the study’s own size cutoffs, the average nodule detection rate per round of screening was 20%. Most studies reported that more than 90% of nodules were benign.
The NSLT found that the rate of positive screening tests was 24.2% with low-dose CT and 6.9% with radiography over all three rounds. A total of 96.4% of the positive screening results in the low-dose CT group and 94.5% in the radiography group were false-positive results. The numbers of false-positive results are likely to be higher in never-smokers, in whom the incidence of cancer is lower. In lower-risk populations, the incidence of false-positive results from screening would be expected to be even higher than that reported in the NSLT.
A detected nodule will likely trigger further imaging. The frequency of further CT imaging among screened individuals ranged from 1% to 44.6%. The frequency of further positron emission tomography (PET) imaging among screened individuals ranged from 2.5% to 5.5% in the NLST. Findings could also result in invasive evaluation. In the NLST, 1.2% of patients who were not found to have lung cancer underwent an invasive procedure such as needle biopsy or bronchoscopy, and 0.7% of patients who were not found to have lung cancer had a thoracoscopy, mediastinoscopy, or thoracotomy. Invasive nonsurgical procedures occurred in 73% of patients with benign lesions in the NLST. Anxiety and unnecessary interventions and complications from these procedures should all be weighed against the potential benefit of LDCT screening.
Radiation Exposure
The risk of radiation-induced cancer from lung CT screening is small. Most relevant, however is the relative magnitude of the potential absolute benefit from screening compared to the risk of induced cancer. The effective dose of radiation of LDCT is estimated to be 1.5 mSv per examination, although there is substantial variation in actual clinical practice. Diagnostic chest CT (∼8 mSv) or PET–CT (∼14 mSv) to further investigate detected lesions accounts for most of the radiation exposure in screening studies. It is estimated that NLST participants received approximately 8 mSv per participant over 3 years, including both screening and diagnostic examinations (averaged over the entire screened population).
Estimates of harms from radiation come from several official bodies and commissioned studies, based on dose extrapolations from atomic bombings and also many studies of medical imaging. Brenner et al. previously estimated the risk of radiation-induced lung cancer mortality for smokers aged 50, and suggested that lung cancer mortality would need to be reduced by at least 5% to outweigh these risks. This figure is likely to be higher for screening at younger ages because the radiation risks will be higher, due to the longer time available to develop a radiation-induced cancer, while the absolute benefit will be lower because lung cancer incidence rates are lower.
Using the NLST data, these models predict that approximately one cancer death may be caused by radiation from imaging per 2500 persons screened, compared to a benefit of prevention of one cancer death per 320 persons screened reported by the NSLT investigators. The benefit, therefore, in preventing lung cancer deaths in NLST is greater than the radiation risk—which only becomes manifest 10 to 20 years later.
Younger individuals or those with lower risk of developing lung cancer have less favorable trade-offs. Radiation-induced cancer risk estimates from lung CT screening are not currently available for never smokers, or for screening before age 50. Preliminary modeling studies suggest that potential risks may vastly outweigh benefits in nonsmokers or those aged 42 years or younger.
Berrington de Gonzalez et al. conducted a study to estimate the potential risk of radiation-induced lung cancer from three annual lung CT screens for asymptomatic individuals starting at age 30, 40, and 50 years. They estimated the level of screening efficacy that would be required to outweigh these risks of radiation exposure (Table 75.1). The risk estimates were developed for never smokers and current smokers. For women, they also estimated the risk of radiation-induced breast cancer. They used the Cancer Prevention Study II to estimate the lung cancer rates for never smokers. For current smokers, they used the Bach lung cancer risk model, assuming a 40-cigarettes-per-day smoking history, which has been recently validated using data from the Alpha-Tocopherol, Beta-Carotene trial.
Table 75.1 Percentage reduction in lung cancer mortality needed to outweigh risk of radiation by smoking status, age, and gender (Source: Adapted from Berrington de Gonzalez et al. J Med Screen. 2008;15:153–158. Reproduced with permission of Sage).
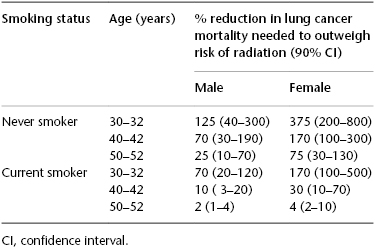
Table 75.1 summarizes the mortality reduction required to outweigh the radiation risks for each man and woman depending on their age and smoking status. As illustrated in Table 75.1, for a woman who is 50 years old and a never smoker, screening has to have a benefit of approximately 75% reduction of mortality to justify the risk of radiation exposure. This is clearly unlikely given the results of the NSLT that demonstrated only a 20% reduction in mortality in the higher-risk screened population.