Methodological and Practical Challenges for Personalized Therapies in Non-Small-Cell Lung Cancer
The University of Texas MD Anderson Cancer Center, Houston, TX, USA
Introduction
Lung tumors are the result of a multistep and complex process in which normal lung cells accumulate genetic and epigenetic abnormalities and evolve into cells with malignant biological capabilities. The identification of an oncogene, or other specific products required by the tumor cells for sustained growth (oncogene addiction), followed by administration of a specific inhibitor to the target are the basis of personalized cancer treatment. Recent advances in understanding the complex biology of lung cancer, particularly oncogene addictions, have provided new treatment targets and allowed the identification of subsets of tumors with unique molecular profiles that can predict response to therapy in this disease. The successful development of personalized therapy depends on the identification of a specific molecular target that drives cancer growth and the subsequent validation of a clinically applicable biomarker molecular test. In this process, the analysis of molecular changes is becoming increasingly important and poses multiple challenges to adequately integrate both routine histopathology analysis and molecular testing into the clinical pathology diagnosis for selection of therapy. In non-small-cell lung carcinomas (NSCLCs), this is best exemplified by treating patients with tyrosine kinase inhibitors (TKIs) when their tumors harbor activating epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene rearrangements.
In this chapter, there is description and discussion of important methodological and practical issues that represent significant challenges for personalized therapy of NSCLC. Several of the recommendations discussed in this chapter have been obtained from the recently published evidence-based Molecular Testing Guideline for Selection of Lung Cancer Patients for EGFR and ALK Tyrosine Kinase Inhibitors by the College of American Pathologists (CAP), International Association for the Study of Lung Cancer (IASLC), and Association for Molecular Pathology (AMP).
1. Which molecular test should be performed in a lung tumor specimen?
In lung adenocarcinoma, EGFR mutation and ALK and ROS1 fusions should be tested to select targeted therapy against these three targets. A similar recommendation is valid for mixed tumors with an adenocarcinoma component and for NSCLC not otherwise specified (NOS) (Figure 74.1). In squamous cell carcinoma, although there are some promising molecular targets (e.g., DDR2 and FGFR1), there is no current validated molecular testing to be recommended.
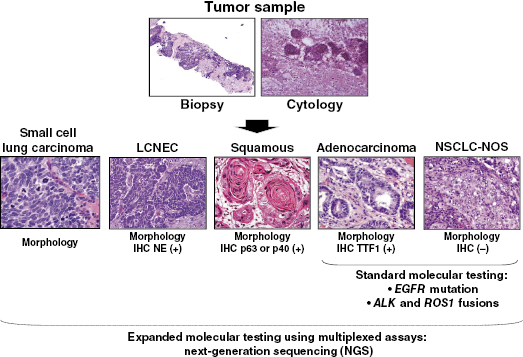
NSCLC represents over 80% of lung cancers. Adenocarcinoma (40%) and squamous cell carcinoma (30%) are the most frequent histologies (Figure 74.1), but there are also less frequent types, including large-cell, adenosquamous (a mixture of adenocarcinoma and squamous cell carcinoma differentiations), and sarcomatoid carcinomas. NSCLC NOS corresponds to tumors in which adenocarcinoma, squamous cell carcinoma, and neuroendocrine differentiation are not detected by histology and immunohistochemical analyses.
Molecular Targets in Lung Adenocarcinoma
At least two different major pathways have been identified in its pathogenesis, a smoking-associated activation of the KRAS signaling, and non-smoking-associated activation of the EGFR signaling (Table 74.1). Lung adenocarcinomas arising in never or light smokers are characterized by significantly higher frequencies of a series of targetable oncogene abnormalities, including EGFR tyrosine kinase (TK) domain activating mutations and EML4–ALK (2;5)(p23q35) translocation. Recently, two additional potentially targetable gene translocations, ROS1 (6q22; SLC34A2–ROS1 and CD74–ROS1) and KIF5B–RET (10p;11q)(p11.22; q11–21), have been identified in lung adenocarcinoma from never and ever smokers.
Table 74.1 Summary of molecular abnormalities associated with lung adenocarcinoma and squamous cell carcinoma histologies.
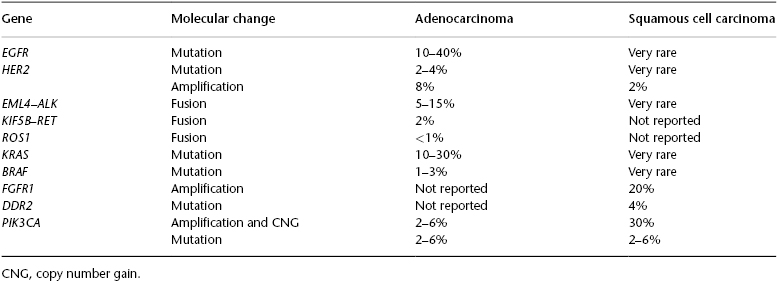
Mutations of EGFR occur in ∼24% of adenocarcinomas and up to 60% in tumors from never smokers. The mutations are limited to the first four exons of the TK domain (exons 18–21). The most frequent mutations are in-frame deletions in exon 19 (44% of all mutations) and missense mutations in exon 21 (41% of all mutations). In addition, in-frame duplications and insertions occurring in exon 20 have been described in about 5% of the mutant cases, and rare missense mutations occur in multiple sites. EGFR mutations occur predominantly in adenocarcinoma (∼20–48%; vs. other NSCLC histologies: ∼2%), and are more frequent in never smokers (54%; vs. ever smokers: 16%) and female patients (49%; vs. male patients: 19%). EGFR mutations comprise the most important criterion to select patients for EGFR TKI therapy in lung cancer.
Aberrant ALK expression has been identified in a subset (∼6%) of lung adenocarcinomas, and this abnormality consists in the formation of a fusion transcript with cell-transforming activity, which is the product of a inverted translocation of the EML4 gene located at chromosome 2p21 and the ALK gene located at 2p23. EML4–ALK translocations have multiple distinct isoforms (up to nine) with demonstrated transforming activity. EML4–ALK translocation has been detected, particularly in patients with never or light smoking history, and has been associated with young onset of tumor. Histologically, EML4–ALK-rearranged adenocarcinomas have been described to have a predominantly solid pattern with signet ring cells, but also combined acinar and cribiform patterns have been described in these tumors. ALK fusion is the criterion to select patients for ALK-targeted therapy (crizotinib) in lung cancer. In addition, tumors with ROS1 fusion have also shown a higher response to therapy with crizotinib.
Molecular Targets in Squamous Cell Carcinoma
This tumor type has been histologically and molecularly less studied than the adenocarcinoma histology (Table 74.1). Squamous cell carcinoma also harbors genetic abnormalities resulting in activation of oncogenes, including EGFR-vIII (deletion of exons 2–7) and DDR2 mutations, and FGFR1 (8p12) gene amplification. However, these molecular targets are still under clinical investigation. Amplification of FGFR1 (chromosome 8p11–12) is a driver event in NSCLC, predominantly in squamous cell carcinoma histology subtype (∼20%) compared with adenocarcinomas (1–3%). Mutations of the TK DDR2 have been described in 4% of lung squamous cell carcinomas. Tumors with FGFR1 amplification and DDR2 mutation have demonstrated some sensitivity to FGFR1 TKI and dasatinib, respectively.
2. When should a lung tumor specimen be tested for molecular markers?