T-Cell Depletion in Allogeneic Hematopoietic Cell Transplantation
Memorial Sloan Kettering Cancer Center and Weill Cornell Medical College, New York, NY, USA
Introduction
Graft-versus-host disease (GVHD) contributes significantly to transplant-related morbidity and mortality (TRM), with an associated mortality rate of 10–25% after hematopoietic cell transplantation (HCT). The risk of grade II–IV acute GVHD in recipients of human leukocyte antigen (HLA)-matched related donor (MRD) and matched unrelated donor (MUD) grafts approaches 35–50% and 40–70%, respectively, with the use of current immunosuppressive regimens. Long-term immunosuppression is required in 30–40% of patients who develop GVHD. The recognition that GVHD was mediated by donor-derived T-cells led to preclinical and clinical exploration of T-cell depletion to reduce the risk of GVHD. The use of ex vivo T-cell-depleted (TCD) grafts has significantly reduced the risk of GVHD without the need for posttransplant immunosuppression. In this chapter, we will focus on the use of ex vivo T-cell depletion or CD34 selection of the graft rather than the use of in vivo antibodies such as antithymocyte globulin (ATG) or alemtuzumab.
1. What methods are used for T-cell depletion of the graft?
Although the most commonly used method of T-cell depletion currently relies on positive selection of CD34+ hematopoietic stem cells from the graft, over the years several different approaches have been used (Table 66.1). These differ primarily in the use of negative versus positive selection. Negative selection can be achieved through either physical methods such as counterflow elutriation or soybean lectin agglutination (SBA) and sheep red blood cell (sRBC)–rosette depletion (E-rosetting), or immunological methods using monoclonal antibodies. Monoclonal antibodies can be used with or without complement, or can be conjugated to toxins. Antibodies can have narrow specificities, such as T10B9 targeting the α/β T-cell receptor (TCRαβ), or broad specificities, such as a combination of antibodies targeting CD2, CD4, and CD8. More recently, removal of T-cells from the graft has been achieved through positive selection of CD34+ cells using immunomagnetic beads. In earlier studies, CD34+ selection of peripheral blood stem cells (PBSCs) was performed on the ISOLEX 300i magnetic cell selection system (Baxter, Deerfield, IL), followed by E-rosetting. Current studies are using immunomagnetic beads on the CliniMACS cell selection system (Miltenyi Biotech, Gladbach, Germany) for CD34+ selection. The CliniMACS system can also be used to negatively select grafts through depletion of CD3 and CD19 cells or depletion of TCRαβ T-cells.
Table 66.1 Examples of methods used for T-cell depletion of stem cell grafts.
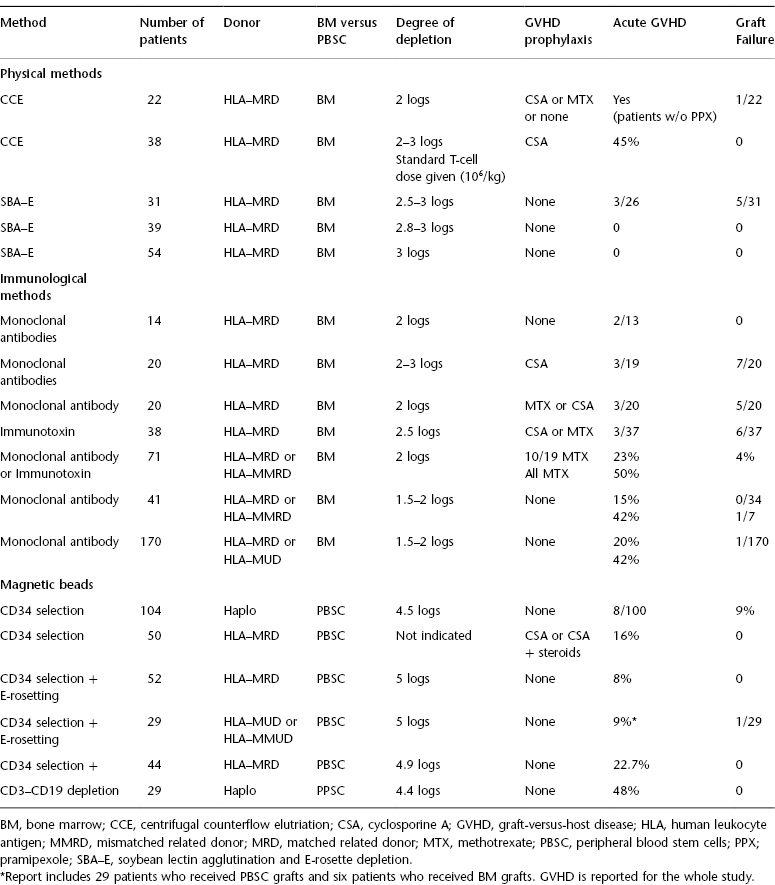
When assessing data from clinical trials reporting the use of TCD grafts, it is critical to review the specific approach used. In particular, the specific populations being removed from the graft (T-, B-, and NK-cells and all nonhematopoietic stem cells), the degree of T-cell depletion, the stem source (bone marrow vs. peripheral blood stem cells), the degree of HLA match (matched vs. mismatched vs. haploidentical), and the use of posttransplant GVHD prophylaxis (standard vs. reduced vs. none) can have a significant impact on clinical outcomes. The degree of T-cell depletion is a particularly critical factor. In recipients of TCD marrow grafts from HLA-identical donors, the risk of GVHD was shown to increase if the graft contained >1 × 105 T cells/kg. For example, the CliniMACS system can achieve a 5-log reduction in T-cells, whereas the ISOLEX 300i system achieves a 3.5-log reduction, requiring additional T-cell depletion through E-rosetting. Therefore, the differences between T-cell depletion methods can have a significant impact on clinical outcomes, including the risk of graft failure, GVHD, and relapse.
2. What is the impact of T-cell depletion on engraftment?
A potential consequence of T-cell depletion is a higher risk of graft rejection. In fact, early studies of T-cell depletion were associated with higher rates of graft rejection than those observed among recipients of conventional grafts, with reported graft failure rates as high as 27%. These clinical results confirmed preclinical data that donor-derived T-cells facilitate engraftment. Following modifications of the conditioning regimen, and in particular the use of ATG to promote engraftment, several centers have reported consistent engraftment with TCD grafts using a variety of approaches for T-cell depletion, including CD34 selection.
3. What is the impact of T-cell depletion on GVHD?
The main goal of using a TCD or CD34-selected graft is to reduce the risk of both acute and chronic GVHD. This result has been achieved in most studies, although to varying degrees. The risk of GVHD generally has correlated with the extent of T-cell depletion. Studies using methods that result in a 1–2 log depletion of T-cells generally have rates of acute GVHD from 15% to as high as 50% when mismatched donors are used. This includes studies in which posttransplant GVHD prophylaxis is given. In contrast, the risk of both acute and chronic GVHD decreases significantly when the degree of T-cell depletion is 3-log using bone marrow or 4–5-log using PBSCs. For example, studies performed at Memorial Sloan Kettering Cancer Center (MSKCC) that achieved a 5-log reduction in T-cells using CD34 selection followed by E-rosetting have reported incidences of acute GVHD (limited to grade II) of 8%, and chronic GVHD of 9% in recipients of matched related grafts, and incidences of acute and chronic GVHD of 9% and 29%, respectively, in recipients of MUDs. None of the patients received GVHD prophylaxis beyond T-cell depletion of the graft. Interestingly, in the setting of MRDs, ATG was not required for engraftment when patients were conditioned with total-body irradiation (TBI), thiotepa, and fludarabine. Results from single-center studies have been validated in a Blood and Marrow Transplant Clinical Trials Network multicenter study (BMT CTN 0303), in which 44 patients with acute myeloid leukemia (AML) in first or second remission (CR1 or CR2, respectively) were conditioned with TBI, thiotepa, and cyclophosphamide with rabbit ATG followed by a TCD PBSC allogeneic graft from an HLA-identical sibling donor. The incidence of acute GVHD grade II–IV was 22.7%, and the incidence of extensive chronic GVHD was 6.8% at 24 months. Importantly, these results are in the setting of PBSC grafts. Finally, this approach has also been used successfully with grafts from haploidentical donors. Aversa et al. (2005) reported a series of 104 patients with acute leukemia (AML: 37; acute lymphoblastic leukemia (ALL): 37) who received CD34-selected PBSC grafts from haploidentical donors after conditioning with TBI, thiotepa, fludarabine, and ATG. Graft failure was observed in 7 of 101 evaluable patients, and acute GVHD developed in 8 of 100 patients.
4. What is the impact of T-cell depletion on relapse?







