Allogeneic Hematopoietic Cell Transplantation in Multiple Myeloma
1Medical College of Wisconsin, Milwaukee, WI, USA
2Fox Valley Hematology Oncology, Elgin, IL, USA
Introduction
Due to our inability to cure multiple myeloma (MM) with current therapies, including autologous stem cell transplantation (auto-SCT), there has been a sustained interest in allogeneic hematopoietic cell transplantation (allo-HCT) given its use of a myeloma-free donor cell graft and the possibility of a donor-driven, immune-mediated graft-versus-MM effect. Based on reporting to the Center for International Blood and Marrow Transplant Research (CIBMTR), fewer than 300 patients underwent an allo-HCT for MM between 2010 and 2011.
The Evolution of the Allogeneic Approach in MM
• What are the risks of allo-HCT in MM, and how has the field evolved over time?
Historically, allo-HCT preceded by classic myeloablative conditioning was of limited applicability except in very young patients, and it was associated with unacceptable treatment-related mortality (TRM) of 40–60%. The allo-HCT arm of the US Intergroup S9321 study was terminated after an early TRM of 53% was observed. However, allo-HCT survivors demonstrated a plateau survival at 22% with no late relapse events, indicating a likely cure.
The advent of nonmyeloablative and reduced-intensity conditioning (NST–RIC) approaches in the 2000s led to more patients receiving allo-HCT using these regimens. A CIBMTR analysis demonstrated a major practice switch to NST–RIC-based allo-HCT with concomitant reduction in the numbers of myeloablative allografts. Lower-intensity conditioning also resulted in expanded eligibility, with older MM patients receiving allo-HCT and increasing numbers of allo-HCT performed after auto-SCT in a tandem auto-SCT–allo-HCT fashion. Interestingly, survival after allo-HCT did not improve over time since the decline in TRM was negated by an increase in relapse risk in later years.
Several phase II studies were reported utilizing the strategy of an initial auto-SCT followed (usually 3–6 months later) by lower-intensity allo-HCT using NST–RIC. The rationale was to uncouple myeloablation (achieved by the auto-SCT) from the immune-mediated benefits of the allo-HCT approach. Excellent short-term (24-month) outcomes could be achieved with TRM ranging from 11% (for related donor grafts) to 26% (for unrelated donor grafts). Median event-free survival (EFS), however, was disappointing at 3 years, and the median time to progression was 5 years. Five-year overall survival (OS) and EFS were 64% and 36%, respectively, with patients transplanted within 10 months of initial therapy having 5-year OS of 69% and EFS of 37%. The lack of an apparent cure with ongoing late relapses was disappointing.
Randomized Trials
• Are there modern randomized prospective trial data regarding the role of upfront allo-HCT in MM?
Several randomized trials (summarized in Table 60.1) have attempted to evaluate the tandem auto-SCT–allo-HCT approach versus tandem auto-SCT in the upfront transplant setting. Bruno et al. (2009) randomly assigned patients (on sibling donor availability) to allo-HCT versus a second tandem auto-SCT after initial induction and a first auto-SCT. Eighty patients with an HLA-identical sibling were assigned to 2 Gy (Gray) total body irradiation (TBI)-based allo-HCT, and 82 patients to a second auto-SCT. After a follow-up of 7 years, median OS was not reached (P = .02) and PFS was 39 months (P = .02) in the 58 patients who received an allograft whereas OS was 5.3 years and EFS 33 months in the 46 who received two autografts. In those achieving complete remission (CR) after allo-HCT, 53% were in continuous CR compared with 19% in CR following tandem auto-SCT. This was the first randomized study that showed an advantage for allo-HCT over auto-SCT in MM and indicated that CR achieved after allo-HCT was durable.
Table 60.1 The autologous stem cell transplantation (auto-SCT)–allogeneic hematopoietic cell transplantation (alloHCT) approach versus tandem auto-SCT in the upfront transplant setting.
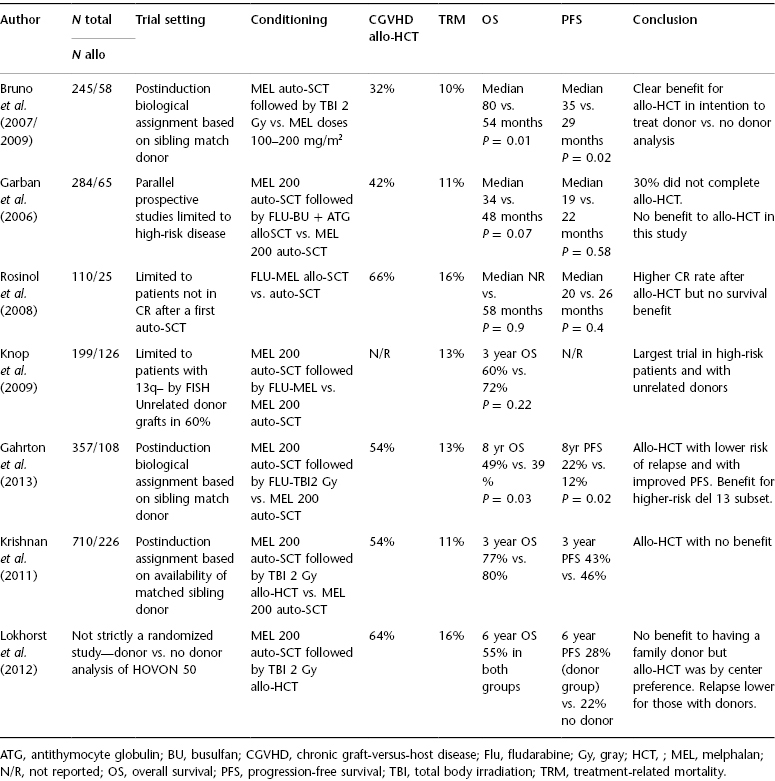
Other prospective randomized studies have demonstrated discordant results. In the BMT CTN (Blood and Marrow Transplant Clinical Trials Network) 0102 multicenter study, tandem auto-SCT and auto-SCT–allo-HCT arms were similar for the primary endpoint of 3-year progression-free survival (PFS) (46% in the tandem auto-SCT group vs. 43% in the other). Higher-risk patients also did not benefit from the auto-SCT–allo-HCT approach in terms of 3-year PFS.
Another randomized European study has now, with extended 8-year follow-up, continued to show improved PFS and OS for the tandem auto-SCT–allo-HCT approach versus tandem auto-SCT. At 96 months, PFS and OS were 22% and 49% versus 12% (P = 0.027) and 36% (P = 0.030) favoring tandem auto-SCT–allo-HCT. Relapse was lower in the allo-HCT cohort (60% vs. 82%; P = 0.0002), although TRM was higher in this cohort. Interestingly, patients who relapsed and progressed following allo-HCT had a significantly higher OS than the patients who relapsed after tandem auto-SCT. The graft-versus-MM effect is thought to have played a major role in this phenomenon.
Meta-analyses of the published allo-HCT versus auto-SCT studies have concluded that while CR rates are higher for allo-HCT, a consistent survival benefit cannot be demonstrated. In summary, while allo-HCT induces high CR rates and provides superior antirelapse potential compared with auto-SCT, TRM rates remain prohibitive. In the absence of a clear-cut survival advantage across studies and with recent improvements in induction and maintenance therapy, some experts have suggested the end of allo-HCT in MM. However, MM is still incurable with novel induction followed by auto-SCT, and while two randomized studies have shown a survival benefit for allo-HCT, no study has suggested an inferior outcome with allo-HCT. It is also notable that these allo-HCT trials were performed in patients who had not received highly effective modern induction regimens.
• What accounts for the discrepancy between randomized studies?
The discordant outcomes are likely due to variations in the conditioning regimens employed, patient selection, MM risk profile, length of follow-up (shorter for the negative BMT CTN study), and the use of agents such as ATG (antithymocyte globulin), which may reduce the potential for graft-versus-MM effect.
• How strong is clinical evidence for a graft-versus-MM effect?
Several lines of evidence exist. Myeloma (idiotype)-specific CD4 T-cell response could be transferred from an immunized marrow donor to patients in early studies. The success of donor lymphocyte infusions (DLIs) in patients with residual or progressive MM after allo-HCT is corroborative. Although the durability of responses after DLI is modest, the occurrence of GVHD (acute or chronic) after DLI seems to be the most powerful predictor of a response. The prospective BMT CTN 0102 study and a retrospective CIBMTR study also found that the occurrence of chronic GVHD after allo-HCT correlated with freedom from progressive MM. In vitro or in vivo T-cell depletion has been associated with higher relapse rates and a need for DLI after allo-HCT in MM.
• Who are the patients for whom current standard approaches are ineffective and allo-HCT is reasonable?
• Define ultra-high-risk MM.
The persistent risk of higher TRM and the prospect of chronic graft-versus-host disease (GVHD) limit the use of allo-HCT to younger patients with MM. Even in younger patients with MM, prospective data from the Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and the German Multicenter Myeloma Group (GMMG) study HOVON-65–GMMG-HD4 indicate that with bortezomib-based induction followed by single or tandem auto-SCT and consolidation–maintenance strategies, durable long-term remissions can be achieved. Allo-HCT should therefore be limited to those with exceptionally high risk of early relapse with current standard approaches.
The term “ultra-high-risk MM” is used to characterize patients who by baseline risk stratification have an estimated median survival of 24 months or less. This subgroup includes those presenting with International Staging System (ISS) stage 3 disease and with specific genetic abnormalities such as deletion 17p, immunoglobulin heavy-chain gene translocations t(4;14) or t(14;16), and chromosome 1q21 amplification (>3 copies). Since the outcomes for these patients remain poor despite the best available alternative therapies, allo-HCT may be considered despite the risk of higher TRM and GVHD. In the uncommon situation of a relatively young (<50 years old) ultra-high-risk patient, even higher-intensity myeloablative conditioning followed by allo-HCT is reasonable as long as patients are aware of their unfavorable prognosis and are willing to accept the risks of conditioning.
A subanalysis of the HOVON-65 International Stage (ISS GMMG-HD4 study identified a high-risk subgroup (comprising approximately 18% of patients) characterized by the presence of del(17p13), t(4;14), and 1q21 (>3 copies) and a high ISS score of II or III. Median PFS for this group was only 18.7 months. Those relapsing early (≤18 months from transplant) after modern novel agent-based induction and auto-SCT represent another group where allo-HCT may be considered.
Practice Point
In patients with ultra-high-risk MM, it is our policy to offer upfront allo-HCT to patients if they are eligible for allo-HCT by virtue of young age, performance status, and comorbidity score.
• When should allo-HCT be offered in the disease course of MM—upfront or at relapse?







