Hematopoietic Cell Transplantation in Indolent Non-Hodgkin’s Lymphoma
1Medical University of South Carolina, Charleston, SC, USA
2Stanford University Medical Center, Stanford, CA, USA
1. Is there a role for autologous hematopoietic cell transplantation (HCT) as a consolidative strategy for follicular lymphoma (FL) in first remission?
The management of advanced FL remains ill-defined partly due to the large number of therapeutic options available. Patients with advanced FL and their physicians have numerous treatment options, including single-agent or combination chemotherapy, monoclonal antibodies (mAbs, including radioimmunoconjugates), and radiotherapy. These therapies have the potential to prolong progression-free survival (PFS), but none is known to provide a cure. As a result, there is no single standard front-line or second-line therapy, and no consensus as to the optimal sequence of the therapies.
High-dose therapy (HDT) with autologous HCT (auto-HCT) has been explored as consolidative therapy in first remission (CR1) and in the setting of disease relapse. Three randomized phase III trials from Europe evaluated the efficacy of auto-HCT vis-à-vis conventional chemotherapy followed by interferon-ɑ maintenance (Table 51.1). Two of these three trials demonstrated a PFS advantage with auto-HCT. However, there was no overall survival (OS) difference between HCT and conventional therapy in all three studies. In fact, there was a significantly increased incidence of therapy-related malignancies in the HCT arms that mitigated the PFS advantage conferred by HCT. However, these trials were conducted in the pre-rituximab era, which limits the relevance of these data in current practice. This is further supported by fact that the PFS reported in the auto-HCT arms of the GELA (Groupe d’Etude des Lymphomes de l’Adulte) and GLSG (German Low-Grade Lymphoma Study Group) studies is comparable to the PFS of FL patients treated with rituxan RTX-containing front-line therapy in the contemporary cooperative group trials.
Table 51.1 Randomized trials of chemotherapy versus autologous HCT (previously untreated).
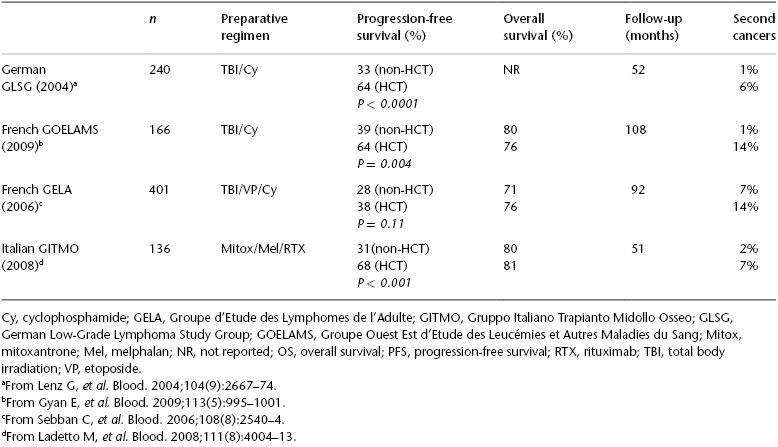
After the availability of rituximab (RTX), a randomized trial was conducted by the Gruppo Italiano Trapianto di Midollo Osseo (GITMO) and Intergruppo Italiano Linfomi (IIL) that compared auto-HCT with standard therapy in high-risk FL patients. A total of 136 patients received upfront R-CHOP therapy and were then randomized to receive additional RTX or auto-HCT. With a median follow-up of 51 months, the results were consistent with those of the previous studies: an improved 4-year event-free survival (EFS) (61% versus 28%; P < .001) without any OS advantage with HCT. This trial also demonstrated that molecular remission was the strongest predictor of outcome and that the HCT arm had a higher incidence of molecular remission compared with conventional chemotherapy (80% versus 44%, respectively). Patients who relapsed after receiving chemotherapy were crossed over to the HCT arm. This group of relapsed FL patients (n = 28) had a 3-year EFS and OS of 68% and 81%, respectively, at a median follow-up of 30 months. Two meta-analyses also confirmed the improved PFS with auto-HCT for FL in first remission, without any OS advantage.
Based on the available data, auto-HCT as consolidation therapy is not recommended for FL patients in CR1. However, because the majority of the data come from trials that did not include RTX, longer follow-up is necessary in patients who received RTX with initial therapy and in the peri-HCT period to determine the efficacy of auto-HCT as a consolidative strategy for patients with FL in CR1.
2. What is the optimal timing for autologous HCT in the treatment of relapsed follicular lymphoma?
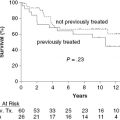
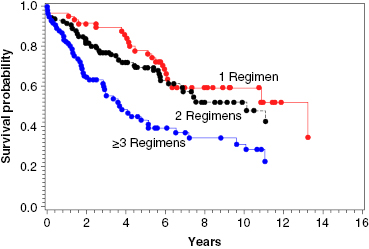
Auto-HCT has a more defined role in patients with relapsed FL than for those in CR1. Several studies, prospective and retrospective, have examined the outcomes after auto-HCT for relapsed FL, and they have reported high response rates, with 5-year PFS ranging from 40% to 50%, and one study reporting a 10-year PFS of 48%. With regard to prognostic factors, patients who have not been heavily pretreated (i.e., ≤3 prior regimens), those with chemosensitive disease, and those with a lower-risk Follicular Lymphoma International Prognostic Index (FLIPI) score at the time of auto-HCT had better OS (Figure 51.1).
The European Blood and Marrow Transplant Group (EBMT) reported a retrospective analysis of the outcomes of 693 FL patients who underwent auto-HCT. The 10-year PFS and OS were 31% and 52%, respectively. The post-HCT relapse rate was 54%, with relapse occurring at a median of 1.5 years (range: 0.08–13.5 years) after auto-HCT. The nonrelapse mortality (NRM) was 9%. Multivariate analysis revealed inferior survival with older age, chemoresistant relapse, and use of total body irradiation (TBI)-based conditioning. Sixty-four patients (9%) developed a second primary malignancy at a median of 7 years after HCT. Another German retrospective series of 241 FL patents who underwent auto-HCT showed a 10-year PFS and OS of 49% and 75%, respectively, with a follow-up of 8 years. A total of 47% patients relapsed at a median of 20 months (range: 2–128 months) after HCT. Five patients in this series developed a therapy-related malignancy.
The only randomized trial that prospectively addressed the role of auto-HCT compared with standard therapy in relapsed FL patients was the EBMT-sponsored CUP (chemotherapy vs. unpurged arm vs. purged arm) trial. A total of 140 FL patients with chemosensitive relapse (after salvage chemotherapy) were randomized (n = 89) to receive further chemotherapy, auto-HCT with a purged graft, or auto-HCT with an unpurged graft. The results demonstrated a PFS advantage and suggested an OS advantage of auto-HCT over conventional chemotherapy, with a 4-year OS of 46% for the chemotherapy-only arm, versus 71% for the unpurged and 77% for the purged auto-HCT arms, with no significant benefit observed for those patients who underwent purging. The sample sizes in the HCT arms were too small to quantify the effect of ex vivo purging. There are two caveats to this trial: the first is inadequate power, as the trial closed early owing to slow accrual; and, second, RTX was not part of standard therapy when the trial was conducted, which makes the results less relevant today. Nonetheless, the results of the CUP trial are in line with those observed in the phase II studies, which included larger numbers of patients.
The GELA and Groupe Ouest Est d’Etude des Leucémies et Autres Maladies du Sang (GOELAMS) studies retrospectively evaluated the outcomes after auto-HCT compared with conventional salvage in 175 patients with FL in first relapse. Of these, 40% had had prior RTX and 40 patients (25%) underwent auto-HCT. With a median follow-up of 31 months, the 3-year OS was significantly superior in patients who proceeded to HCT compared with patients who did not (92% vs. 63%; P = 0.0003). In addition, this analysis did not show any effect of front-line RTX on the overall outcome. As with any retrospective study, the superior outcomes with HCT may be the result of selection bias, because only patients responding to salvage therapy underwent HCT.
In summary, FL patients who are beyond CR1 but are chemosensitive, do not have bone marrow (BM) involvement, and have a good performance status are optimal candidates for auto-HCT. However, for patients with limited disease involvement or an early stage at relapse, consideration should be given to involved-field radiotherapy with or without chemotherapy.
3. Does the “graft-versus-lymphoma” effect exist with allo-HCT for patients with follicular lymphoma?