Derek Gordon and Florence Brown
Note: with the exception of Hypurin Bovine Lente, bovine and porcine insulins have been excluded from the table.
Insulin type
Onset of action
Peak action
Duration
Soluble (human)
Human Actrapid
30 minutes
2–4 hours
5–8 hours
Humulin S
Insuman Rapid
Short-acting analogues
Insulin lispro (Humalog)
5–10 minutes
30–90 minutes
2–4 hours
Insulin aspart (NovoRapid)
Insulin glulisine (Apidra)
Isophane (Human)
Humulin I
2 hours
6–12 hours
18–24 hours
Insulatard
Insuman Basal
Insulin Zn suspension
Hypurin Bovine Lente
2 hours
8–12 hours
30 hours
Long-acting analogues
Insulin glargine (Lantus)
1–3 hours
Flat with no peak
24 hours
Insulin detemir (Levemir)
1–3 hours
6–7 hours
20–24 hours
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


5. The person with type 1 diabetes
CHAPTER 5. The person with type 1 diabetes
Introduction 97
Epidemiology 97
The clinical presentation of type 1 diabetes 98
The aims of insulin therapy 99
Currently available insulins 102
Inhaled insulins 105
Formulations of injected insulin 105
Insulin regimens 106
Hypoglycaemia 110
Insulin adjustment 115
Nurse prescribing 117
Insulin injection technique and equipment 118
Conclusion 119
References 119
The history of the discovery of insulin in 1921 is one of intrigue, personality clashes and betrayal, and of a medical student on a summer job placement achieving the Nobel Prize. Without insulin, newly diagnosed children and young adults with diabetes faced a slow, wasting disease that could be treated only by a starvation diet and that led to an inevitable, early death. The discovery of insulin offered a chance of life to those previously living without hope.
EPIDEMIOLOGY
Type 1 diabetes accounts for approximately 10% of all people with diabetes and affects 10–20 million people worldwide. Type 1 diabetes generally affects people under the age of 40 years and 40% develop it before the age of 20 years. One of the most striking characteristics of type 1 diabetes is the large geographic variability in incidence. The Scandinavian countries and the Mediterranean island of Sardinia have the highest incidences in the world, whereas Oriental populations have the lowest incidences. The reasons for such geographical differences are not known.
THE CLINICAL PRESENTATION OF TYPE 1 DIABETES
Tom is an 18-year-old who has presented to his GP with a 3-week history of thirst. He was drinking up to 2 litres of carbonated drinks per day. He had noticed that he was passing much more urine than normal and was getting up through the night on at least three occasions. During this period of time his weight had fallen by about 4 kg. He had become increasingly tired and lethargic and had also noticed that his vision had become blurred. He also admitted to painful cracking of the foreskin. Glycosuria was confirmed as 2% and there was 3+++ of ketonuria using urine testing strips. The diagnosis of type 1 diabetes was confirmed by measurement of plasma glucose of 23.0 mmol/L. Tom was referred immediately by telephone to the local consultant diabetologist, who arranged to see him that day and insulin treatment was started.
People presenting with type 1 diabetes typically give a short and dramatic history of polydipsia, polyuria and weight loss. The lack of insulin causes a rise in blood glucose, which acts as an osmotic diuretic causing polyuria and polydipsia. In an attempt to provide energy, the body mobilises its glucose and fat reserves and, in so doing, switches into ketone production (see Chapter 1). This accounts for the acute weight loss, tiredness and lethargy. Left untreated, the person would develop diabetic ketoacidosis and coma. Nowadays, this is less frequently seen due to heightened awareness of the early diabetic symptoms by healthcare workers and the public in general.
Tom’s blurred vision was due to the presence of glucose in the lens of the eye. This causes alteration in the shape of the lens and subsequent blurring of vision due to altered refraction. This corrects itself as, with treatment, blood glucose levels return to normal, but it can take up to 6 weeks before the blurring disappears. People who are newly diagnosed with diabetes should be advised not to get their eyes tested for glasses for up to 3 months from diagnosis or 2 months from the time that their diabetes is stable.
The presence of sugar in the urine encourages the development of penile thrush, as in Tom’s case. Reducing glycosuria will eliminate the growth of organisms. In the meantime, however, Tom will also require appropriate antifungal treatment.
At the hospital clinic, Tom would be seen by the diabetes specialist team and have blood samples taken for glucose, urea and electrolytes including bicarbonate, liver function tests, full blood count and glycated haemoglobin (HbA1c). The possibility of diabetic ketoacidosis (DKA) needs to be considered. DKA is a medical emergency and requires hospital admission.
The dietician would assess Tom’s current diet and recommend dietary changes in the light of his history, lifestyle and estimated energy consumption. The DNS would start insulin therapy and teach Tom how to perform home blood glucose monitoring (HBGM) and arrange to see him frequently to continue his stabilisation and education. Tom would then enter a full education programme involving all the members of the healthcare team. This might continue over several weeks or months (see Chapter 11).
Question ‘Will my children get diabetes?’
A frequently asked question following a diagnosis of diabetes is whether other family members will be affected. In areas of the world where the risk of diabetes is moderate (e.g. the UK) the risk of developing type 1 diabetes by age 20 years is approximately 1 : 300. The risk is increased to 1 : 50 for children of women with type 1 diabetes and as high as 1 : 15 if a person’s father has type 1 diabetes. It is also estimated that by the age of 60 years approximately 10% of first-degree relatives will develop type 1 diabetes. If a child has type 1 diabetes then there is a 1 : 10 chance that another sibling will also be diagnosed with type 1 diabetes.
LESS TYPICAL PRESENTATIONS
Type 1 diabetes can affect people over the age of 40 years and can even occur in the elderly. It is now recognised that it can present with less acute symptoms. It can sometimes be difficult to decide in both young and older people whether they have type 1 or type 2 diabetes at the time of presentation. When the type of diabetes is in doubt, the diagnosis of type 1 diabetes should be considered if:
▪ ketonuria is detected, or
▪ weight loss is marked, or
▪ the person does not have features of the metabolic syndrome (see Chapter 4) or other contributing illness.
THE AIMS OF INSULIN THERAPY
▪ To preserve life.
▪ To relieve the symptoms of hyperglycaemia, i.e. polydipsia, polyuria, lethargy and weight loss.
▪ To restore ‘normal metabolism’.
▪ To prevent diabetic ketoacidosis.
The development of diabetes is associated with many subtle changes in metabolism, for example, lipids, blood clotting and connective tissue biochemistry. Many of these secondary changes in metabolism are responsible for the long-term complications of diabetes. The Diabetes Control and Complications Trial (DCCT) (DCCT Research Group 1993), as well as a number of smaller studies, have clearly demonstrated the beneficial effects of improved diabetic control in preventing the microvascular complications of diabetes (Wang et al 1993).
THE DIABETES CONTROL AND COMPLICATIONS TRIAL
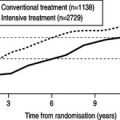
Nearly 1500 people with type 1 diabetes were recruited from 29 diabetic clinics across the USA to take part in the Diabetes Control and Complications Trial (The DCCT Research Group 1993). People were randomly allocated to receive up to 10 years’ conventional or intensified treatment. Conventional treatment consisted of one or two daily injections of insulin and education about diet and exercise. People were reviewed every 3 months. Intensified treatment aimed for long-term near-normoglycaemia with at least four home blood glucose assessments a day, three or more insulin injections daily and monthly visits to a clinic with further advice freely available by telephone between clinics.
The trial was halted prematurely after people had been followed-up for periods ranging between 3 and 9 years. Those individuals in the intensively treated group maintained significantly better metabolic control throughout the study period. However, despite the intensive treatment, only 5% of this group attained the goal of near-normoglycaemia throughout the study.
Nevertheless, the study dramatically demonstrated that improving diabetic control was associated with a significant reduction in the risks of developing diabetic complications or their progression. The reduction of 2% in HbA1c in the intensively treated group of individuals was associated with:
▪ 76% reduction in the risk of newly developing retinopathy and 54% reduction in the progression of retinopathy
▪ 39% reduction in the incidence of microalbuminuria and 54% reduction in progression to proteinuria
▪ 60% reduction in development of neuropathy.
This improvement was apparent at all levels of metabolic control. In other words, a reduction in HbA1c from 16% to 14% was associated with the same improvement in outcome as a reduction from 10% to 8%. Nevertheless, it remains the case that those people with poorest control have the greatest risk of complications.
Targets for glycaemic control
The DCCT has implied that there is no threshold figure of HbA1c below which complications do not occur. However, on the basis of epidemiological studies in the DCCT and UK Prospective Diabetes Study (see Chapter 4), the microvascular risk appeared to be low once an average HbA1c was around 7.0–8.0% while macrovascular risk continued to fall with HbA1c levels down to 6.0–7.0% (DCCT standardised). This has led the National Institute for Health and Clinical Excellence (NICE) to make the following recommendations (NICE 2004):
▪ Adults with type 1 diabetes should be advised that maintaining a DCCT-harmonised HbA1c below 7.5% is likely to minimise their risk of developing diabetic eye, kidney or nerve damage in the longer term.
▪ Adults with type 1 diabetes who want to achieve an HbA1c down to, or towards 7.5% should be given all appropriate support in their efforts to do so.
▪ Where there is evidence of increased arterial risk (identified by raised albumin excretion rate, features of the metabolic syndrome, or other arterial risk factors) people with type 1 diabetes should be advised that approaching lower HbA1c level (for example 6.5%) might be of benefit.
Tight (meaning the achievement of normoglycaemia) metabolic control has its downsides. People in the DCCT intensively treated group gained weight and experienced three times as many episodes of severe hypoglycaemia as the conventionally treated group. It should be remembered that many people with type 1 diabetes fear severe hypoglycaemia more than complications in middle or late life.
Tight metabolic control might not be appropriate for the following groups of people:
▪ people with loss of warning signs of impending hypoglycaemic attack
▪ young children, particularly under the age of 7 years, when hypoglycaemia can be associated with damage to the developing brain
▪ frail, elderly people or those with limited life expectancy in whom the rigors associated with close metabolic control, would not be appropriate
▪ people who have limited abilities to treat hypoglycaemia independently.
Self-monitoring of blood glucose
Self-monitoring of blood glucose was an integral part of the intensive treatment group in the DCCT. However, self-monitoring is only likely to affect blood glucose control when used to inform self-management of diabetes. In clinical practice there is often little relationship between frequency of blood glucose self-monitoring and frequency of insulin dose self-adjustment (Gordon et al 1991).
NICE has therefore made several recommendations, including the following:
▪ Self-monitoring of blood glucose levels should be used as part of an integrated package that includes appropriate insulin regimens and education to help inform choice and achievement of optimal diabetes outcomes.
▪ Adults with type 1 diabetes should be advised that the optimal frequency of self-monitoring will depend on:
▪ the characteristics of their blood glucose control
▪ the insulin regimen
▪ personal preference in using the results to achieve the desired lifestyle.
Chapter 7 further expands on the evidence base for blood glucose monitoring.
Insulins with different chemical structures, depending on their source and manufacture, are available:
▪ Bovine (Wockhardt UK)
▪ Porcine (Wockhardt UK)
▪ ‘Human’ (NovoNordisk, Lilly, Sanofi-Aventis)
▪ Insulin analogues (NovoNordisk, Lilly, Sanofi-Aventis).
ANIMAL INSULINS
The chemical structures of these insulins differ from human insulin by a few amino acids. Bovine insulin differs from human insulin in three of its 51 amino acids, and is thus more likely to cause antibodies to be formed against it. Porcine insulin differs in only one amino acid residue (alanine in place of threonine).
‘HUMAN’ INSULINS
There are two different methods by which ‘human’ insulin is produced. Porcine insulin can be chemically altered by replacing the alanine amino acid with threonine (enzymically modified pro-insulin: emp insulin).
Alternatively, ‘human’ insulin can be produced by introducing the gene for human insulin into bacteria or yeast (prb: proinsulin recombinant bacteria, or pyr: proinsulin recombinant yeast insulins). The organisms are cultured in huge vats and the insulin is harvested and purified.
Following the introduction of ‘human’ insulins there was much concern about altered warning signs of hypoglycaemia and an increased reported incidence of severe hypoglycaemic episodes with the newer insulins. However, a subsequent literature survey of 39 studies and 12 epidemiological reports concluded that there were no significant differences in the physiological responses to hypoglycaemia or the frequency of hypoglycaemic episodes between human and porcine insulins (Jogensen et al 1994). However, a number of individuals with long-term diabetes remain unhappy about taking human insulin. Healthcare professionals should remain receptive to these views and individuals should be able to continue to use porcine or bovine insulins.
INSULIN ANALOGUES
An analogue is a chemical with a similar, but not identical, molecular structure to another chemical. Insulin analogues have been produced in order to develop insulins which have novel properties. Box 5.1 lists the insulin analogues that are now available in the UK.
Box 5.1
Insulin analogues currently available in the UK
Short-acting analogues
▪ Insulin lispro (Lilly)
▪ Insulin aspart (Novo Nordisk)
▪ Insulin glulisine (Sanofi-Aventis)
Long-acting insulin analogues
▪ Insulin glargine (Sanofi-Aventis)
▪ Insulin detemir (NovoNordisk)
The currently available short-acting insulin analogues have been created by making amino acid substitutions at one or more sites in the insulin molecule. Insulin molecules normally aggregate together forming hexamers, that is, six molecules of insulin loosely bound together. In this form the hexamer is too large to cross from the subcutaneous site into the circulation. The hexamers must first separate into dimers and then into single insulin molecules before absorption into the bloodstream can occur. This process takes some time and delays the onset of action of the conventional insulins. However, the analogue insulins have been designed to prevent this aggregation and formation of hexamers. This means that when the short-acting insulin analogues are injected into the subcutaneous space they are absorbed much more rapidly.
The short-acting insulin analogues therefore have a more rapid onset of action and a shorter duration of action than the conventional insulins (Mudaliar et al 1999, Nielsen et al 1995; Table 5.1). These insulins therefore have the advantage that they can be injected immediately before, or indeed immediately after, eating. Their peak serum concentration coincides with the postprandial rise in blood glucose and a meta-analysis of several studies has shown that rapid-acting insulin analogues are more effective than short-acting ‘human’ insulin in improving postprandial glucose control, without an increase in the rate of hypoglycaemic episodes (Davey et al 1997). The shorter duration of action should help to prevent hypoglycaemic episodes occurring before the next meal. People perceive an improvement in their quality of life with rapid-acting analogues due to the increased flexibility of injection times and less frequent hypoglycaemic episodes.
Characteristics of the long-acting insulin analogues
Insulin glargine has also been produced by altering the amino acid sequence of human insulin. The substitution of an asparagine amino acid by glycine and the addition of two arginine amino acids to the end of the insulin β chain results in the insulin becoming more soluble at acid pH. When the insulin is injected into the relatively alkaline, subcutaneous space the insulin glargine (Lantus, Sanofi-Aventis) forms microprecipitates. These tiny crystals are absorbed slowly and at a constant rate into the blood stream. The characteristics of its action profile are shown in Table 5.1. Insulin glargine has a ‘peakless’ action and is, therefore, ideal insulin to act as basal therapy.
Insulin detemir has a fatty-acid side chain added to the insulin molecule. This allows it to bind to albumin, which again slows its rate of release into the blood and produces a flat and ‘peakless’ blood concentration curve following injection. Insulin detemir has a shorter duration of action than insulin glargine (see Table 5.1) and may require to be injected twice daily for people with type 1 diabetes.
A further property of the long-acting insulin analogues is the reproducible blood profile from one injection to another. Insulin detemir has a lower coefficient of variation (23–27%) than insulin glargine (36–48%) and both are lower than isophane insulin (46–68%) (Vague et al 2003). (Coefficient of variation is a mathematical measure of variability.) People develop more confidence in their insulins when the effect on blood glucose levels becomes more reproducible from day to day.
Alternatives modes of delivery of insulin (without requiring painful, skin injections) have been sought for many years but with little success until recently. Insulin in a dry powder form, which allows the insulin particles to be delivered to the lung alveoli, has now been developed. The insulin thus inhaled can be rapidly absorbed across the thin alveolar walls into the circulation. Exubera (Pfizer and Sanofi-Aventis) is the first inhaled insulin to come to market.
When Exubera is inhaled, blood insulin levels rise rapidly and in a similar fashion when tested against the short-acting insulin analogue, insulin lispro. Its duration of action is, however, prolonged and simulates that of the older soluble insulins (Rave et al 2005). Several studies have now demonstrated the efficacy of inhaled insulin in people with both type I and type 2 diabetes (Hollander et al 2004, Quattrin et al 2004, Skyler et al 2005).
Inhaled insulin is contraindicated for people who smoke and should not be introduced until cigarette smoking has ceased for at least 6 months. It is also not suitable for people with poorly controlled asthma or chronic obstructive airways disease. Inhalation is associated with coughing at the time of inhalation but this does not appear to be a major problem. Inhaled insulin is also associated with deterioration in lung function as measured by forced expiratory volume in 1 second (FEV1) and carbon monoxide diffusing capacity (DLCO). However, these changes are small, of dubious clinical significance, and thought to be of a temporary nature.
Freemantle and colleagues (Freemantle et al 2005) have demonstratedincreased acceptability of inhaled insulin over conventional insulin delivery when offered to people with type 2 diabetes. It is likely that inhaled insulin delivery will prove popular with people who have diabetes. However, long-acting, background insulin will still be required to be given as subcutaneous injections.
FORMULATIONS OF INJECTED INSULIN
All conventional insulins are currently available in three broad types of formulation, classed according to their duration of action (see Table 5.1).