CHAPTER 4. The person with type 2 diabetes
Derek Gordon
The metabolic syndrome 68
Why treat type 2 diabetes?68
Self-monitoring in type 2 diabetes 71
Non-drug treatment of type 2 diabetes 71
Pharmacological treatment of type 2 diabetes 74
Insulin therapy 84
Drugs of the future 88
Medications altering glucose tolerance and drug interactions 90
Conclusion 92
References 93
A WORLDWIDE EPIDEMIC
Type 2 diabetes has reached epidemic proportions both in developing countries and in the developed world. It is estimated that throughout the world there are currently 150 million people with diabetes, and that number will double by 2025. Globally, 97% of these people will have type 2 diabetes, although in the industrialised countries this figure falls to 90%.
In the UK, it is estimated there will be 2.88 million people with type 2 diabetes by 2010. Data from eight European countries indicate that the mean cost per patient with diabetes is US$2928 annually (1999 values), and the proportion of healthcare spending on diabetes ranges from 1.6% to 6.6%, depending on the country.
Changes in human lifestyle over the past century have precipitated this dramatic increase in the incidence of diabetes. Increasing prosperity, ready access to food – much of which is now ready-made, convenience food – and a more sedentary way of life have resulted in an explosion in the incidence of obesity. The Nurses’ Health Study found that in 91% of people with type 2 diabetes the condition could be attributed to a body mass index (BMI) > 23, lack of exercise, unhealthy diet, smoking and abstinence from alcohol (Hu et al 2001).
Obesity is associated with increased resistance to insulin action and the development of type 2 diabetes. Insulin resistance also increases with age and an ageing population has also contributed to the numbers of people with diabetes.
It is now recognised that insulin resistance is associated with a cluster of factors known as the ‘metabolic syndrome’. Overall obesity, and in particular central obesity, dyslipidaemia [characterised by elevated levels of triglycerides and low levels of high-density lipoprotein (HDL) cholesterol], hyperglycaemia and hypertension are common traits that, when they occur together, constitute the metabolic syndrome. The metabolic syndrome is very common, affecting about 24% of US adults between the ages of 20 and 70 years. People with the syndrome are about twice as likely to develop coronary heart disease as people without it.
It should, therefore, be recognised that type 2 diabetes is usually part of a more complex metabolic disorder and that treatment of the blood glucose levels should not be undertaken in isolation. It is important to tackle all aspects of the metabolic syndrome, that is, obesity, dyslipidaemia and hypertension. This chapter deals with the management of diabetes control; Chapter 8 covers the management of other cardiovascular risk factors.
THE PREDIABETIC STATE
The development of diabetes takes many years. The earliest feature in most people is the development of insulin resistance. The pancreas can initially compensate by producing more insulin and blood glucose levels can be maintained within normal limits. Eventually, however, the pancreas can no longer produce sufficient insulin to overcome the worsening insulin resistance and blood glucose levels begin to rise. At this stage people will have impaired glucose tolerance. Eventually the pancreatic beta cells begin to fail and insulin levels fall. This results in higher blood glucose concentrations and the development of diabetes.
THE SPECTRUM OF TYPE 2 DIABETES
Type 2 diabetes is therefore the result of both insulin resistance and insulin lack, hence people with type 2 diabetes form a classic spectrum of disease. At one end of the spectrum are obese people who have predominantly insulin resistance, at the opposite end are non-obese individuals with predominant insulin insufficiency.
WHY TREAT TYPE 2 DIABETES?
There are several reasons for treating the blood glucose concentrations in diabetes. Hyperglycaemia is associated with symptoms of lethargy, thirst and frequency of urination, including nocturia. Poorly controlled diabetes can lead to vaginal or penile thrush. Treatment of diabetes can relieve the symptoms of hyperglycaemia.
It had also been assumed for many years that control of blood glucose levels would prevent some of the complications of diabetes. This was demonstrated, for people with type 2 diabetes, when the results of the UK Prospective Diabetes Study (UK Prospective Diabetes Study (UKPDS) Group, 1998a and UK Prospective Diabetes Study (UKPDS) Group, 1998b) were published.
UK PROSPECTIVE DIABETES STUDY
The UK Prospective Diabetes Study (UKPDS) was a large, multicentre, clinical trial that started in 1977 and reported its final results in 1998. The aims of the study were to identify whether, in people with type 2 diabetes, tight metabolic control reduced the macrovascular and microvascular complications and whether any particular form of therapy was best in achieving tight control. In addition, the study aimed to demonstrate whether tight control of blood pressure prevented macro- and microvascular complications. The blood pressure control part of the study will not be discussed in this chapter.
During the course of the study, several UKPDS reports were published that revealed aspects of the epidemiology and natural history of type 2 diabetes. The study followed 3867 people with newly diagnosed type 2 diabetes. Individuals were randomly assigned to conventional treatment (the aim of which was to maintain fasting blood glucose at < 15 mmol/L) or intensive treatment (the aim of which was to achieve fasting blood glucose levels < 6 mmol/L).
People were allocated to treatment by diet, a sulphonylurea or insulin therapy. In addition, a small subgroup of obese individuals was allocated to initial treatment with metformin.
Results of the UKPDS
The study demonstrated that in both the conventional and intensive treatment groups, there was a steady increase in fasting blood glucose and glycated haemoglobin (HbA1c) over the period of the study. Type 2 diabetes is therefore a progressive disease associated with worsening metabolic control over a number of years (Fig. 4.1). This implies that metabolic control can be maintained in type 2 diabetes only by progressive increases in doses of medications and the utilisation of multiple therapies, including insulin. The different treatment modalities showed little difference on their effect on fasting blood glucose or HbA1c. Only people treated with chlorpropamide had significantly improved metabolic control when compared with other sulphonylureas or insulin.
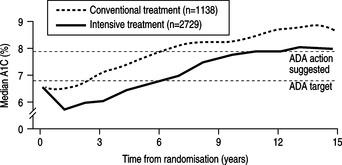 |
| Fig. 4.1HbA1c rises with time in both the conventional and the intensively treated groups of patients with type 2 diabetes.Reprinted from UKPDS (1998a), with permission from Elsevier. |
The intensively treated group showed an 11% reduction in HbA1c when compared to the conventional group (HbA1c 7.0% compared to 7.9%). For every 1% increment in HbA1c the study demonstrated a:
▪ 21% increased risk of any diabetes-related endpoint
▪ 21% increased risk of any diabetes-related death
▪ 14% increased risk of a myocardial infarction (MI)
▪ 37% increased risk of any microvascular complication.
Furthermore, the UKPDS (1998a) demonstrated that benefits could be achieved by reducing HbA1c results no matter how low they were to start with; in other words the lower the HbA1c the better. Microvascular risk became low when HbA1c reached levels of 7–8% whereas macrovascular risk continued to fall, down to HbA1c levels of 6–7%.
The difference in HbA1c results between the conventional and intensive treatment groups was associated with a significantly reduced frequency of microvascular endpoints; the reduction in diabetes-related mortality or macrovascular endpoints did not quite reach statistical significance. However, the group of obese people who were treated intensively with metformin showed a significant reduction in cardiovascular risk.
Metformin was the only treatment that was not associated with weight gain and raised circulating concentrations of plasma insulin. Sulphonylureas and insulin treatments were associated with increased plasma insulin concentrations and insulin resistance, and this might therefore have accounted for their poorer influence on long-term cardiovascular morbidity.
SELF-MONITORING IN TYPE 2 DIABETES
There is no evidence that blood or urine testing improves HbA1c, body weight or the incidence of hypoglycaemic events in people with type 2 diabetes. The National Institute for Health and Clinical Excellence (NICE) does not recommend the use of self-monitoring as a stand-alone intervention in people with type 2 diabetes (NICE 2004). Rather, it recommends that self-monitoring be taught only if the purpose or need for it is clear and agreed with the individual with diabetes.
Self-monitoring can be useful in the period immediately after diagnosis, when people can learn the effects of various foodstuffs on their blood or urine glucose levels. This can sometimes be effective in encouraging alterations to diet. It can be useful to teach self-monitoring to people in whom oral hypoglycaemic agents are failing; this can act as an incentive to improve control. Also, if they subsequently require insulin treatment they will already have mastered the technique prior to starting insulin. No doubt there are other situations when self-monitoring can be useful. However, prolonged self-monitoring with no clear purpose is an expensive practice that may not be beneficial. See Chapter 7 for further elaboration.
NON-DRUG TREATMENT OF TYPE 2 DIABETES
DIET
Dietary review is fundamental to the management of the person with type 2 diabetes. The basic tenets of dietary management should therefore be known to the primary healthcare team so that preliminary advice can be given in the community at the time of diagnosis (see Chapter 6). However, few doctors or nurses have adequate training to provide complete dietary advice and all newly diagnosed people should receive advice from a dietician.
Well over 50% of all newly diagnosed people with type 2 diabetes are overweight (BMI 25–30 kg/m2) or obese (BMI > 30 kg/m2). The cornerstone to their diet is an individualised reduced-calorie diet (see Chapter 6). Eating less reduces energy intake and allows weight reduction. Weight reduction will improve insulin resistance and allow the person to utilise his or her own insulin production more effectively.
When diet succeeds, the benefits are evident. Blood glucose, lipids and blood pressure fall. However, the response to diet is often disappointing. Most diabetic clinics in general practice or hospital lack the resources required to allow frequent dietary review and people often lack motivation or find it impossible to alter deeply ingrained eating habits. The UKPDS found that only 16% of newly diagnosed people with type 2 diabetes achieved near-normal fasting blood glucose concentrations after 3 months of dieting. Those people with the highest fasting blood glucose levels at the time of diagnosis were least likely to achieve good diabetic control by diet alone (UKPDS 1990).
EXERCISE
Several studies have demonstrated the benefit of lifestyle intervention on the development and management of diabetes. Modest weight loss and increased physical activity have been shown to cause substantial reduction in the risk of developing type 2 diabetes (Helmrich et al 1991, Manson et al 1991). For each 500-kcal increment in weekly energy expenditure, the risk of developing type 2 diabetes can be reduced by 6%.
Decreased cardiac risk
Exercise confers the well-established benefit of improved insulin sensitivity for people with or without diabetes. As with weight loss, improved insulin sensitivity is associated with reduction in blood pressure and improvement in lipid profiles, thus reducing cardiovascular risk. Improved fitness and exercise are associated with a reduction in coronary disease in the general population, but evidence for such a decrease among people with diabetes has not appeared.
Weight loss
The addition of exercise training to a conventional diet has been shown to be beneficial (Blonk 1994) with people showing greater weight loss and improved HbA1c. However, weight loss tends to be modest in most people and only a minority maintain their weight loss over a 3-year period (Stevens et al 2001).
Improved glucose utilisation
Increased insulin sensitivity allows glucose to enter muscle cells more efficiently both acutely and chronically with exercise. Improvements in glucose tolerance testing have been shown in type 2 diabetes after as little as 1 week of aerobic training. Increased insulin sensitivity begins to decline however, in as little as 1 or 2 days without exercise.
Exercise can therefore improve diabetic control and allow lower doses of oral hypoglycaemic drugs or insulin. However, the exercise must be maintained and this requires both self-motivation by the individuals and continuing encouragement by their families, friends and health carers. The optimal methods for maintaining motivation are still to be identified.
Bernard is a 52-year-old taxi driver who was recently diagnosed as having type 2 diabetes when he attended his GP complaining of tiredness and thirst. A random blood glucose was found to be 23 mmol/L. He has been overweight for many years and currently weighs 107 kg (BMI 34 kg/m2). When working, he eats erratically from cheap hot-food stalls. He smokes 30 cigarettes per day and never takes any exercise. His GP estimates his weekly alcohol intake at approximately 28 units. His mother and two brothers have type 2 diabetes.
Despite adherence to a restricted-calorie diet for 6 months and some increase in his exercise activities, Bernard continues to complain of thirst and frequency of urination during the night. His weight has fallen by 4 kg but his HbA1c remains elevated at 9.2%. His GP decides to start him on metformin.
The finding of a random blood glucose above 11.1 mmol/L in a person with osmotic symptoms confirms diabetes. Bernard’s elevated blood glucose might be the cause of his lethargy. In addition to his diabetes, his unhealthy lifestyle will further increase his risk of vascular catastrophe in the future unless he makes major alterations to the way he lives.
Bernard’s case history is fairly typical of the person with type 2 diabetes. There is a strong family history of diabetes and he has the associated risk factor of being overweight. He is likely to have other features of the metabolic syndrome; namely hypertension and hyperlipidaemia. His initial management will involve appropriate changes to his lifestyle including diet and exercise.
As Bernard has a sedentary lifestyle, he should be encouraged to increase his exercise activity. It is recommended that all individuals (including those with diabetes) undertake aerobic physical activity on a minimum of 3 days per week with sessions lasting 20–60 minutes. However, our 52-year-old taxi driver might have to start with low intensity exercise for shorter periods of time. In some areas of the UK, general practitioners can prescribe an exercise programme at a local gym. It is important to undertake a pre-exercise assessment of people with diabetes prior to entry into any such programme. Watch for signs and symptoms of peripheral vascular disease, such as intermittent claudication, cold feet, decreased or absent peripheral pulses. Diminished light touch, pin-prick or vibration sense might signal a peripheral neuropathy. Such people might be at risk of foot ulceration if asked to undertake exercise. Eyes should be examined to identify new vessel formation (see Chapter 9).
A history of postural hypotension, hypoglycaemia unawareness, gustatory sweating or impotence might signal the presence of autonomic neuropathy which, if present, could make exercise dangerous because of risks to the heart.
An exercise stress test is recommended for people at risk of heart disease who anticipate engaging in moderate to intense exercise. Among people with diabetes who are considered at risk are those who are older than 35 years, who have had type 2 diabetes for longer than 10 years and those who have had type 1 diabetes for longer than 15 years.
PHARMACOLOGICAL TREATMENT OF TYPE 2 DIABETES
METFORMIN: A BIGUANIDE
In medieval times, the plant, Galega officialis, (goat’s rue or French lilac) was used as a traditional remedy for diabetes in Southern and Eastern Europe. The plant was subsequently shown to be rich in guanidine and in 1918 guanidine was shown to have mild hypoglycaemic effects. However, guanidine was too toxic for clinical use and in the 1950s the biguanide, metformin, was derived and introduced into clinical practice.
Metformin lowers blood glucose concentrations by several mechanisms. Its most important effect is to enhance insulin sensitivity in peripheral tissues, in particular the liver and skeletal muscles. By improving insulin action on the liver, metformin inhibits glucose production and release. It also stimulates insulin uptake by skeletal muscle. It might have direct or indirect effects on reducing appetite (Bailey 1992).
The UKPDS included a subgroup of overweight people who were allocated to treatment with metformin, sulphonylureas (chlorpropamide or glibenclamide) or insulin therapy. Only metformin therapy was associated with improved insulin sensitivity and reduced circulating concentrations of insulin.
Reduced insulin resistance would be expected to improve cardiovascular risk and, indeed, the UKPDS showed that metformin had the greatest effects on any diabetes-related end point, on overall mortality due to any cause and also on the incidence of stroke. The UKPDS also reported that, in overweight people, metformin was not associated with weight gain and produced fewer hypoglycaemic attacks than sulphonylureas or insulin therapy (UKPDS 1998b). It is therefore recommended that metformin is used as first-line treatment in overweight people if lifestyle changes fail to improve diabetic control.
Metformin is likely to reduce blood glucose concentrations by 2–3 mmol/L when prescribed for people in whom diet has failed. This is a comparable blood glucose lowering effect to sulphonylureas. About 30% of people so treated will achieve good diabetic control. However, in a further 5–10% of people per year, metformin treatment will fail to achieve adequate control.
Prescribing metformin
Metformin is available in the UK as 500- and 850-mg tablets. The drug should be taken with meals and the dose increased gradually to lessen side effects. A typical starting dose would be 850 mg once daily or 500 mg once or twice daily. The dosage can be increased in a stepwise fashion over a period of several weeks to prevent adverse drug effects. Garber et al (1997) showed that metformin lowered HbA1c and fasting blood glucose in a dose-related manner, up to 2000 mg per day. Beyond this dosage there is little therapeutic gain but a much higher incidence of side effects.
Side effects of metformin
The most common side effects of metformin are gastrointestinal in origin. About 20% of people experience diarrhoea, flatulence or abdominal pain, whereas others complain of a metallic taste, nausea or anorexia. Although a reduction in appetite might be desirable in the overweight person, for the sake of compliance any nausea or anorexia must be considered seriously. These disturbances are generally transient and can be minimised by starting treatment at a low dosage and taking the drug with food.
Metformin can cause the blood lactate levels to rise. However, if it is prescribed taking into account recognised contraindications, there is no increased risk of serious lactic acidosis or increased levels of lactate compared to other antihyperglycaemic agents (Aguilar et al 1992). Metformin is normally excreted through the kidneys and is contraindicated in people with impaired renal function (e.g. serum creatinine > 130 micromol/L) as this can result in accumulation of the drug in the body, enhancing its effect on lactate production. Liver disease is also a contraindication, because adequate hepatic function is required to metabolise the increased lactic acid. People with cardiac failure perfuse their tissues poorly and the resulting tissue hypoxia causes lactic acid production. It is therefore unwise to prescribe metformin for such individuals. Metformin should be temporarily withdrawn in people undergoing surgery or radiology procedures requiring injection of contrast.
Annie is a 62-year-old housewife who has had type 2 diabetes for 10 years and was initially managed on diet alone. After 3 years her metabolic control deteriorated and as she was obese (BMI 31 kg/m2), metformin was started at an initial dose of 500 mg daily. Gradually, over a number of years, the dose was increased to 1 g twice daily. Over the past 3 months Annie has developed thirst and she has begun to monitor her urine for glycosuria. This has shown persistently elevated results. She denies any recent change to her diet. A clinic HbA1c result is 9.5%, confirming recent poor control.
Before making additions to Annie’s current therapy, it is important to determine if she is actually taking her current prescribed medication. Further dietary assessment should also be undertaken to determine her understanding, identify any necessary changes to be made and to assess her motivation and adherence.
One of the most important findings of the UKPDS was the demonstration that type 2 diabetes is a progressive condition. All modalities of therapy were associated with a relentless rise in HbA1c with duration of disease (see Fig. 4.1). This results in the need for increasing doses of medication and the introduction of multiple therapies to maintain diabetic control. UKPDS (1990) suggested that 3 years after diagnosis approximately half of people controlled on diet alone were failing in their control and required more than one glucose-lowering drug. After 9 years this increased to 75% of people requiring multiple therapies to achieve HbA1c levels averaging 7.0%. Following the failure of metformin to control Annie’s diabetes, the introduction of an insulin secretogogue is recommended (NICE 2004).
INSULIN SECRETOGOGUES
The sulphonylureas
Sulphonylureas have been used to treat type 2 diabetes for more than 50 years. In recent years, understanding of their mode of action has developed. Sulphonylureas bind to cell membrane receptors on the beta cell. This causes the potassium channel across the membrane to close and the membrane to depolarise. In turn, this causes calcium influx and insulin granule release into the extracellular medium. Therefore the effect of sulphonylureas is limited to people with preserved pancreatic cell function. The pancreas can only be squeezed to produce more insulin if there are functioning beta cells there to be squeezed!
It is generally recommended that sulphonylureas are ingested about 30 minutes before meals as this will stimulate insulin production to coincide with the time of eating. This in turn has been shown to reduce the postprandial rise in blood glucose (Melander et al 1989). Most drugs in this group are given twice daily when used at higher doses. The maximum therapeutic effect of most sulphonylureas is achieved at relatively low doses. This is likely to be due to cell receptors for the drug becoming saturated at low doses and further increases in drug dose have no additional effects. A person who shows poor glycaemic control on a dose of gliclazide 160 mg daily or glibenclamide or glipizide 10 mg daily is unlikely to respond optimally to higher doses and a decision to start additional therapy should not be delayed unnecessarily.
Which agent to use?
There are very few studies of the older sulphonylureas such as tolbutamide. For the more recently introduced sulphonylureas there is little evidence that one compound is more effective than another and little to be gained by changing from one agent to another (Gordon 1996).
Nevertheless, there are important differences between the sulphonylureas, in particular their duration of effect (Table 4.1). The shorter-acting sulphonylureas require more frequent dosing, with consequent influence on concordance with treatment (see below). The longer-acting sulphonylureas – glibenclamide and chlorpropamide – are associated with a higher incidence of severe hypoglycaemic attacks (Gordon 1996).
| Drug | Effect duration (hours) | Daily dose (mg) |
|---|---|---|
| tolbutamide | 6–10 | 500–2000 |
| gliquidone | 6–18 | 15–180 |
| gliclazide | 12–20 | 40–320 |
| glipizide | 6–16 | 2.5–20 |
| glimepiride | 12– > 24 | 1–6 |
| glibenclamide | 12– > 24 | 2.5–20 |
| chlorpropamide | 24–72 | 100–500 |
Glimepiride is the most recently introduced sulphonylurea and can be prescribed as a once-daily preparation even at higher dosage (Sonnenberg et al 1997). The starting dose is usually 1 mg daily and this can be increased in 1-mg steps every 1–2 weeks to the usual maximum dose of 6 mg. Glimepiride has been shown to be less likely to cause symptomatic hypoglycaemia than glibenclamide (Dills & Schneider 1996).
These agents tend to cause weight gain and should only be used in obese patients when dietary restriction and metformin therapy have proved inadequate in controlling blood glucose levels.
The most important side effect with sulphonylureas is the development of serious and prolonged hypoglycaemia. For this reason, any person who becomes hypoglycaemic due to a sulphonylurea should be admitted to hospital for at least 24 hours for observation. The elderly are most at risk, probably because of coexisting vascular disease of the brain and heart. Almost all causes of prolonged hypoglycaemia have involved people over the age of 70 years (Ferner & Neil 1988). Of those admitted to hospital, 10% will die and 3% will be left with permanent brain damage. The longer-acting sulphonylureas should be avoided in the elderly.
The short-acting tolbutamide can be used in renal impairment, as can gliquidone and gliclazide, which are mainly metabolised and inactivated by the liver. However, care should be taken to monitor blood glucose levels regularly in order to avoid hypoglycaemia.
Sulphonylurea failure
As type 2 diabetes progresses, beta-cell function deteriorates further and the ability of the sulphonylureas to stimulate insulin secretion also deteriorates. Indeed, by ‘flogging a dying horse’ it is possible that the sulphonylureas might actually hasten the demise of the beta cell. Harrower and Wong (1990) reported 5-year failure rates for gliclazide at 7%, glibenclamide 17.9% and glipizide 25.6%.
SHORT-ACTING INSULIN SECRETOGOGUES
The glinides (meglitinides and D-phenylalanine derivatives)
Glinides, such as repaglinide and nateglinide, are recently introduced insulin secretogogues. Like the sulphonylureas, they bind to the sulphonylurea receptor on the surface of the beta cell. The binding, however, occurs at a different position on the sulphonylurea receptor. Nevertheless, their action is similar to that of the sulphonylureas, causing closure of the transmembrane, potassium channel and subsequent release of insulin from the beta cell.
These drugs are short acting and should be taken before main meals. Their function is mainly to reduce postprandial rises in blood glucose. There is some evidence to support the hypothesis that rapid rises in blood glucose levels are particularly important in the development of diabetic complications. Therefore, drugs that can reduce postprandial glucose excursions might be important in reducing the morbidity and mortality associated with diabetes. However, this theory is at present unproven and controversial.
Repaglinide
Repaglinide is the first meglitinide to become available for clinical use. It is taken before meals at doses of 0.5–4 mg. Studies have shown that repaglinide as monotherapy or in combination with other hypoglycaemic agents such as metformin or rosiglitazone (see below) achieves good metabolic control similar to that achieved by glibenclamide regimens. Repaglinide is associated with less severe and less frequent hypoglycaemic events than sulphonylureas. There is some evidence that repaglinide is less likely to cause weight gain than various sulphonylureas.
Nateglinide
Nateglinide is a d-phenylalanine derivative and is taken before meals at a dose of 60–120 mg. It is not licensed as monotherapy and should be used in combination with metformin. Studies with nateglinide have shown that, compared with glibenclamide, the hypoglycaemic effects of nateglinide are more rapid and greater, while producing less prolonged insulin exposure and less risk of hypoglycaemia. When nateglinide was compared to glipizide, a shorter-acting sulphonylurea, both agents showed similar postprandial glucose excursions. Glipizide produced lower blood glucose levels 4 hours after meals with therefore an increased risk of late hypoglycaemia.
The glinides need to be taken three times a day and therefore compliance with therapy can be reduced. Their exact place in the management of type 2 diabetes remains to be established. They can be particularly suitable for people with erratic lifestyles for whom mealtimes are unpredictable. They might be suitable hypoglycaemic agents for elderly people in whom hypoglycaemia is a concern and for those individuals with renal or mild hepatic failure.
THE THIAZOLIDINEDIONES (ALSO KNOWN AS GLITAZONES)
The mode of action of the thiazolidinediones is complex and not fully understood. They bind to a nuclear protein called peroxisome proliferator-activated receptor-γ (PPARγ), a nuclear receptor found mainly in fat cells but also in muscle and liver. Binding of thiazolidinediones to this receptor protein switches on several genes that are involved mainly with lipid metabolism (Tontonoz et al 1994, Vidal-Puig et al 1997). The net effect of the stimulation of these genes is to reduce circulating levels of fatty acids that are known to be responsible for causing insulin resistance. Also, binding to the PPARγ receptor decreases production of tumour necrosis factor α (TNFα) and of resistin, both of which are involved in causing insulin resistance. The main effect of the thiazolidinediones is therefore to reduce peripheral insulin resistance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree