4 Interventional Radiology in the Treatment of the Cancer Patient
Introduction
Interventional radiology (IR) has a long history of being involved with cancer treatments. Visionary leaders in the field such as Sidney Wallace, MD, began the locoregional therapy of tumors and the use of image-guided therapy for diagnosis and treatment starting with the complications of the disease with obstruction of the biliary tree by pancreatic cancer, hemorrhage occurring from invasion into adjacent structures, to pulmonary emboli prophylaxis with inferior vena cava (IVC) filters. This naturally progressed to therapeutic options for primary and metastatic disease.
Local Tumor Diagnosis
Image-guided biopsy of masses began with fluoroscopically guided pulmonary biopsies. If the mass could be imaged and reliably identified on a chest radiograph, it is likely that it could be successfully biopsied with small-gauge needles, 20 to 22 gauge. Even hilar lesions could be biopsied, but the risks of hemorrhage and pneumothorax were far greater than peripheral lesions. A direct path to the lesion could be plotted using the rotational capabilities of the IR suite and the needle placed into the lesion with a confirmatory orthogonal view. The development of computed tomography (CT) guidance has allowed the precise biopsy of even smaller lesions than seen on fluoroscopy. A fine-needle aspirate should initially be obtained to determine if the lesion is at least suggestive of malignancy. The pathologist could then determine whether more tissue was needed. The availability of a pathologist on site when a biopsy is performed greatly enhances the likelihood of success. In fact, if the pathologist is not in or immediately outside the room, the interventional radiologist needs to insist that they be there. The positive biopsy rate will dramatically increase. Guiding needles slightly larger than the biopsy needle are usually placed so that multiple specimens may be obtained without the danger of repeatedly traversing the pleura. With the advent of genetic marker studies, the demand for larger biopsy specimens has rapidly increased. The use of core needles from 20 to 18 gauge has been helpful in providing these larger specimens. However, the larger the needle, the higher the risk of pneumothorax. Patients must be instructed to stop respiration as the needle is placed through the pleura to lessen the risk of a pneumothorax. However, even when all precautions are taken, the rate of pneumothorax occurring in any image-guided procedure is roughly 30%, of which about 1 in 10 or 3% of all biopsies require chest tube placement. If a pneumothorax is present, the needle should be aspirated as it is removed and much of the air present may be decompressed. Follow-up chest radiographs should be obtained in 1 and 4 hours to determine if the pneumothorax is enlarging. During this observation time, the patient must be admitted to a supervised holding area where they can be monitored for respiratory distress or decreased oxygen saturation and the interventional radiologist immediately available ( Fig. 4.1 ). One of the basic tenets of IR becoming its own specialty must be that we should handle our own complications to the best of our ability and not have to rely on other services to do so. However, know our limitations and use consultations, for example, for cardiac clearance for chest pain by cardiology.
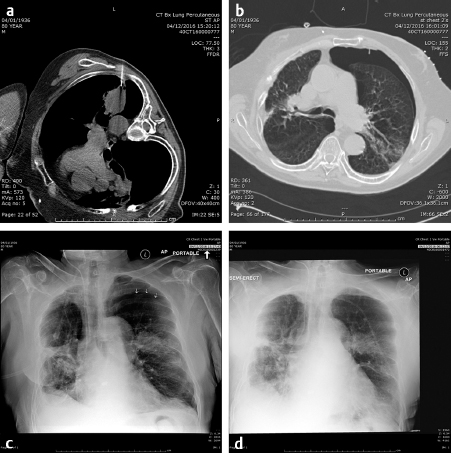
The ability and availability of equipment to place a chest tube must be present where lung biopsies are performed. Since there is little blood in the aspirated air, a simple Heimlich valve apparatus is usually sufficient and the patient is admitted overnight. A chest radiograph is obtained in the morning following admission and, if there is no sign of a pneumothorax, the valve is clamped for 4 hours and a repeat chest radiograph obtained. If there is still no pneumothorax, the tube may be safely removed. If the pneumothorax remains, the tube remains in place for another 12 to 24 hours and the above process repeated. Fortunately, most pneumothoraces resolve after the initial overnight admission. After 48 hours, a bronchopulmonary fistula should be considered and a consult to thoracic surgery made.
The other complication of pulmonary biopsy is hemorrhage. If large vascular structures are avoided and the possibility that the mass may be an arteriovenous malformation considered and excluded, significant hemorrhage is rare. The patient should be warned that small amounts of hemoptysis are expected, but large amounts are unusual and they should go to the nearest emergency room if it occurs. However, now that gene therapy is common, larger amounts of tissue are required for analysis with multiple core biopsies obtained. If numerous core biopsies are obtained, the patient should be watched for several hours for massive hemoptysis and warned that if it should occur, immediate admission to the emergency department is essential or bronchial artery embolization performed. If significant hemorrhage is noted on the CT, the patient should be placed in the decubitus position with the bleeding side down to assist in tamponading the bleed.
Accuracy of needle biopsies varies by the number of passes made into the mass, the experience of the radiologist, and the availability of conscious sedation. With pathologists present to evaluate the specimens obtained, the accuracy is well over 90%. Occasionally, there will be patients who are unable to complete the biopsy even with conscious sedation and general anesthesia must be utilized. This is seen more frequently in pediatric patients and in patients with developmental and mental health issues. Obviously a good relationship with anesthesiology is a tremendous asset. Conversations with the anesthesiologists who cover “out of OR” (operating room) procedures will yield a better working relationship since they are very much out of their element and the necessary adjustments made to the room, the procedural sequence, and equipment availability can be made before the planned procedure and potential emergencies that arise during the procedure. Since general anesthesia usually requires that the patient be admitted to the postanesthesia care unit (PACU), the interventional radiologist should become familiar with the procedures and protocols there.
Once pulmonary biopsies became standard, the areas in which biopsies were requested blossomed to practically every portion of the body from the maxillary sinuses to the phalanges of the toes. This correlated with the growth of CT and ultrasound (US) technology that could produce images quickly. Many have used CT fluoroscopy, but objection to the increased radiation dose to both the patient and the physician has limited its routine utilization. Magnetic resonance imaging (MRI) guided biopsies require specialized nonmagnetic needles and equipment and may be limited to breast biopsies when no other imaging system will visualize the lesion. Additionally, the time required on the MRI is difficult to obtain since the biopsy takes longer than routine scans.
Local Tumor Therapy
From biopsies, the technology developed to ablate tumors. After all, if an interventional radiologist can place a needle into a lesion, isn’t it a short jump to therapy? The initial tumor ablation processes were injection of ethanol or phenol into hepatic lesions. When ethanol comes into contact with cell walls, the walls rupture and the cell is almost instantaneously killed. The results varied since this is treatment of a single lesion with each placement of the needle. It is obviously not practical when numerous lesions are present, but when single inoperable lesions are present, ablative techniques should be considered. The use of ethanol or phenol directly injected into the tumor is unpredictable as to where the liquid travels and how much is enough. Most authors suggest that approximately one-third to one-half the volume of the tumor should be used. Larger lesions are not practical since the fluid may not reach the growing boundary of the tumor and collect inside the necrotic core and too much ethanol may also cause severe intoxication and poisoning. Its use should be limited to experienced physicians and be restricted to lesions that are less than 3 cm in diameter so that no more than approximately 10 to 15 mL of ethanol is utilized. 1
In the early 1990s, the first radiofrequency ablation (RFA) units were produced and received Food and Drug Administration (FDA) clearance, which spurred the development of various array configurations and types of energy applied. RFA is the application of energy in the radiofrequency range (400–1,000 kHz) to a monopolar device so that the atoms within the tumor are either repelled or attracted by the charge on the device. Because the charge on the array is alternating at 60 Hz, the to-and-fro motion of the atoms causes heating due to interatomic friction. When the temperature rises above 46°C, irreversible damage is done to the area treated since the cell walls are disrupted and the cells desiccated. This is also reflected in the electrical resistance. Since the generators are of constant power, as the resistance to electrical current (or in the case of alternating current, the impedance) rises linearly, the current produced by the generator must fall by the square of the current so that the power is constant, producing the so-called “roll-off” of the current applied, shutting off the generator when the current reaches zero. A time–temperature relation exists such that if isolated thermocouples are placed in the tumor along with the array, heating them to a particular temperature for a specific time will give a treatment zone of a given diameter. This is in comparison to the bulk property of the constant power generator that is regulated by the resistance of the treated tumor. Both have their advocates, but the results are comparable. Since these are monopolar arrays, grounding pads must be used. Complications include the following:
Incomplete heating and treatment of the tumor due to heat sink effects from nearby large blood vessels, bowel, organs.
Hemorrhage due to the placement of the probe that is 14 gauge.
Damage to adjacent organs or diaphragm.
Seeding of the tumor along the probe tract (rare).
Burns at the grounding pad site when the pads are not applied securely to the skin and are not observed during the procedure. Since the core body temperature may rise, sweating occurs and may cause the pads to become partially unattached, resulting in a high current density and a burn.
In the lung as in the liver, the largest tumors that can be treated with a very low recurrence rate measure 3 cm in diameter ( Fig. 4.2; Fig. 4.3 ). Larger diameters increase the problems of recurrence since there may be incomplete or variable heating of the tumor even with larger arrays. A margin of at least 5 to 10 mm should be ablated around the tumor to assure that there is complete treatment. Smaller renal cell carcinomas (RCC) may also be treated with RFA. A useful technique is to inject very dilute contrast in the abdomen or retroperitoneum to move structures so that the tumor is accessible to treatment, so-called “hydrodissection” ( Fig. 4.4 ). The same principles apply to microwave ablation, which is ablation utilizing a different portion of the electromagnetic spectrum. It is, however, faster than RFA, but the probes are larger in diameter and the equipment more expensive. If a larger tumor than the antennae on the probe is treated, careful planning must be performed to overlap the fields to ensure that none of the tumor is untreated. This accounts for the higher recurrence rates in the larger tumors. A relatively recent addition to the ablative heating world was the bipolar probe to treat metastatic tumors in the spine and then follow the RFA with polymethyl methacrylate (PMMA) cement placement.
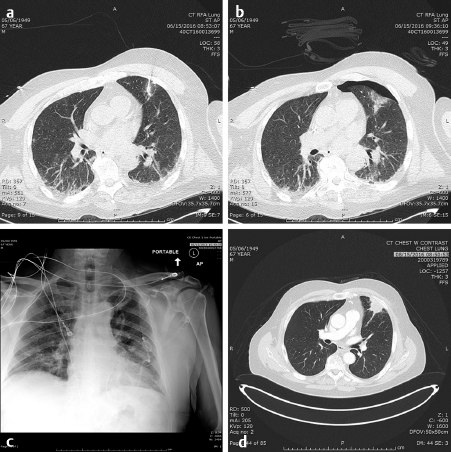
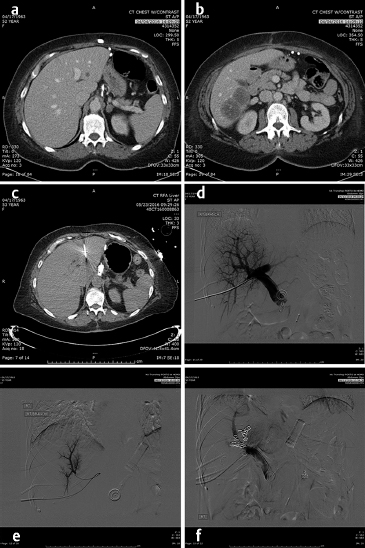
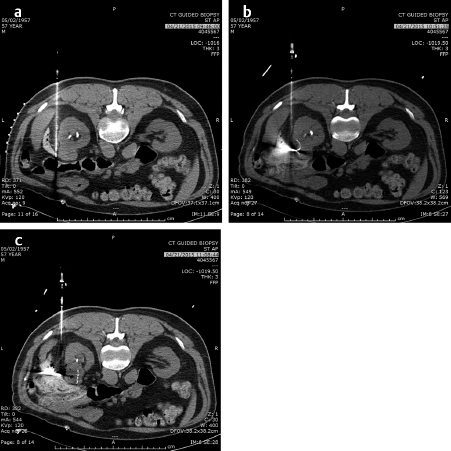
Similarly, the use of cryotherapy to treat tumors was instituted in the late 1980s. This procedure requires the placement of multiple probes through which helium gas under pressure flows. A small chamber at the end of each needle allows the helium to expand and, according to the ideal gas law, the temperature is lowered significantly to well below −100°C, forming an ice ball at the tip of the probe. With multiple probes, the contour of this ice ball can be contoured to the shape of the tumor. This has been utilized with over 90% success in the treatment of renal as well as prostate, lung, bone, and soft-tissue tumors that are 4 cm or less in diameter. while it was used surgically in the liver, the large ice ball could shatter resulting in exsanguination of the patient. The percutaneous use of cryotherapy has been limited in the liver due to the surgical experience. The ice balls may be monitored by CT, US, or MRI imaging. The limitations to the treatment are that it is local and treats one tumor at a time (like RFA), takes somewhat longer than RFA or microwave therapy, and the equipment costs are higher than RFA. However, the results in the kidney and bone show promise. 2 , 3
A new ablative instrument, irreversible electroporation (IRE) has been introduced. This, like so many other ablative techniques, began in the OR with its use in nonoperative tumors. It consists of multiple probes placed around the periphery of the tumor and electrical current of high voltage and low-amperage direct current are delivered between each pair of probes. This is performed under general anesthesia with cardiac gating so that the electrical current is not placed during a sensitive portion of the cardiac cycle. When the tumor cell is treated, the pores in the cell wall remain open and the differential osmolarity between the inside and outside of the cell is eliminated and the cell undergoes apoptosis. 4 For advanced pancreatic primaries, IRE appears to increase overall survival and progression-free survival. 5 IRE has been touted as not being sensitive to the heat sink effect of RFA and being safe for surrounding blood vessels; however, recent articles have noted that the vascular complication rate is near 50% and the effectiveness about the same as RFA for hepatic tumors. 6 The difference between RFA and IRE is the large investment to obtain the equipment, the high cost of the probes, and the extended time required placing the probes precisely under CT guidance.
Venous Access Devices
Probably the most performed procedure in IR today is the placement of venous access devices. It is also the one procedure that links the medical oncologist to IR and forms the basis of a close working and referral relationship. Those practices that have an easy referral system in place will also be able to transform that relationship to the performance of more aggressive and advanced cancer therapy protocols. The skill of the interventional radiologist performing the device placement is also a measure on which the relationship is built. Adherence to the principles of sterile procedures is an absolute necessity since the patients may have these lines for weeks to years. Additionally, the number of central line infections has been a benchmark on which the Centers for Medicare & Medicaid Services grades, judges, and rewards or penalizes the hospital through their reimbursement of services.
Basic laboratory results should be obtained so that coagulation times, platelet count, and the potential for ongoing infections can be evaluated to minimize the risk to the patient. Removing a device recently placed due to infection or a large hematoma arising is inexcusable when the laboratory results can lead to appropriate patient selection and good surgical technique can avoid untoward results. Since there are essentially four to six placement sites available for long-term access device placement, removal of a device and losing one of these sites is a potentially catastrophic problem.
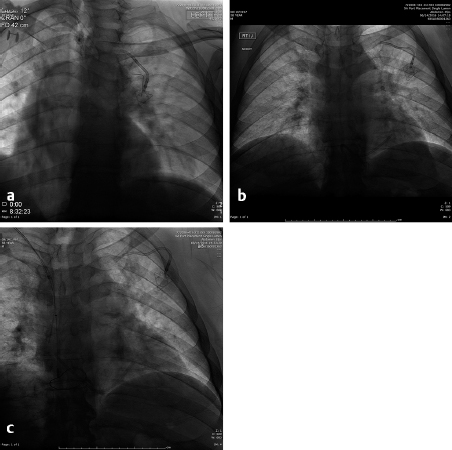
The tunneled central line (the Hickman catheter) was the first to be approved in the 1980s and was almost universally placed in the OR by surgeons. The traditional surgical method of placement of these lines involved a blind puncture of the lateral portion of the left (usually) subclavian vein after local anesthesia and placement of a wire into the superior vena cava (SVC) followed by placement of local anesthesia of a tract in the anterior chest wall that is at least 10-cm long. The catheter is then brought through this tunnel by using a tapered metal tunneler and exits at the venotomy site. A “peel-away” sheath is then placed into the vein and the catheter placed into the venous system as the sheath is torn and removed leaving the outer ends of the catheter outside of the patient’s chest and the tip in the SVC. This placement is then confirmed by a chest radiograph or under fluoroscopy. Surprisingly enough, the acceptable position of the tip of the catheter has been described as essentially anywhere in the SVC as long as blood could be aspirated. Unfortunately, this process included placement of the tip against the right lateral SVC wall where infusions of chemotherapy cannot only cause a fibrin sheath at the tip but a pseudoaneurysm to form in the SVC ( Fig. 4.5 ). The misplacement of the catheter is not the only risk entailed by placing the catheter through a subclavian venous puncture. Others include compression of the catheter so that it “pinches off” through the action of the shoulder muscles and ligaments and eventually results in a broken catheter in the right atrium or pulmonary artery ( Fig. 4.6 ). These catheters are then retrieved by IR, but they are barely visible under fluoroscopy and are difficult to snare with a retrieval device. If they have been present for weeks to months, it is likely they are attached to the endothelium of the heart and the SVC, making the catheter impossible to retrieve. If the catheter is causing arrhythmias or thrombi to form, it must then be removed surgically. The blind puncture of the subclavian vein also brings the risk of pneumothorax, and older sheaths utilized to place the catheter in the venous system risked air emboli since they did not have a diaphragm at the end to prevent the entrance of air. While air emboli can still rarely occur, the interventional radiologist must be familiar with the treatment: place the patient in the left lateral decubitus position and wait until the air dissipates. If the patient is left in the supine position, it is possible that a large air bolus may cause “vapor lock” in the pulmonary artery with dire consequences.
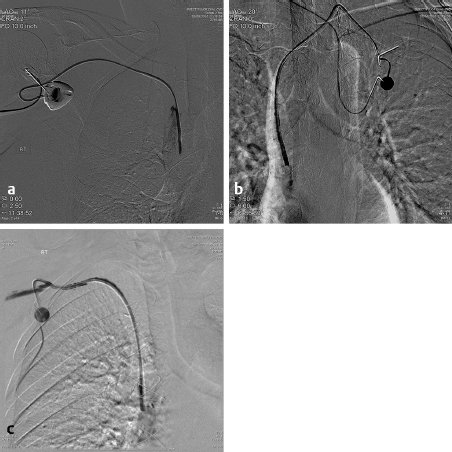
More modern techniques are utilized in the IR suite where, under US guidance, the jugular vein is punctured and a wire placed into the right atrium or inferior vena cava (IVC). This approach avoids the potential “pinch off” from the subclavian approach and the possibility of a pneumothorax. A tunnel is then anesthetized along the chest wall and the catheter brought from the chest to the venotomy site. A diaphragm-containing peel-away sheath is then placed and the catheter placed in the inferior portion of the SVC or the superior portion of the right atrium under fluoroscopic control. The risks from this approach are significantly less and the likelihood of a successful placement is increased. It has also been demonstrated that the results of the jugular approach are superior to the subclavian in duration of patency, infection rate, and complications. 7
The long-term problems with tunneled central lines include the formation of fibrin sheaths at the tip ( Fig. 4.7 ), which can be removed by using a bolus of tissue plasminogen Activator, fibrin stripping by using a retrieval snare and sliding it along the catheter, or, finally, exchange of the catheter by placement of two wires through the lumens, removing the catheter and placement of a new catheter through the same tract. If the tract becomes infected, it may be treated with antibiotics if the organism is not a gram-negative species or the patient has leukemia or lymphoma, saving the venous access site. 8
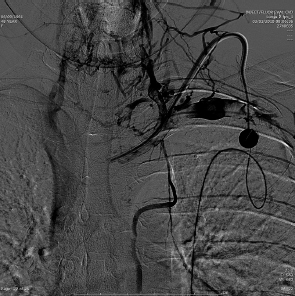
If a catheter is in place for months, it is not uncommon to see central venous stenoses that limit the flow through the catheter and can cause upper extremity edema. These narrowings should be addressed by balloon angioplasty. Since these stenoses are long standing and in the venous system, they have a high fibrin content, while the arterial stenoses are usually due to atherosclerotic disease. It is common to angioplasty these stents and the balloon may have to be inflated for up to 3 minutes. The use of stents is common to treat these stenoses. Uncovered stents are usually utilized so as not to block any small venous inflow ( Fig. 4.8 ).
Implanted Ports
Placement of implantable ports is but a small step beyond the placement of tunneled catheters. A small pocket is made under the selected clavicle just slightly larger than the port to be utilized. A single incision is made and blunt dissection is performed. The catheter may be cut to length and placed before attachment to the reservoir, but most prefer to attach them before placing the catheter into the venous system to prevent air emboli. If the pocket around the port is tight enough, it is unnecessary to place any sutures; however, if a generous pocket is made, suturing the port to the backside of the pocket insures that the port will not rotate and make the access impossible. Ports have been placed and utilized for several years. When removing such long-term ports, it is sometimes extremely difficult when calcification occurs around the catheter and a thick rind of scar tissue envelops the port. Placement of the tip of the catheter is the same for tunneled lines. Ports should be placed within the chest tissue taking into account the amount of breast tissue present so that when the patient stands, the catheter does not automatically withdraw to an unusually high position where it cannot be used successfully. This can be done by pressing the breast tissue inferiorly when the pocket site is selected. Placement of the port in the axilla or deeper than 5 mm beneath the skin surface should be avoided as it inhibits the access of the port. In patients receiving radiation therapy to the chest, the port should be placed on the opposite side to where the radiation is being administered since the port can deflect the photons and influence the dose to the tumor.
There has been a movement to place more peripherally inserted central catheters (PICCs) in the extremities, most commonly for long-term antibiotics but also for chemotherapy. US placement involves the selection of an extremity vein (with or without placement of a tourniquet so that the vein will be enlarged), usually the brachial or basilic. The skin is then anesthetized and the vein entered with a 21-gauge needle, followed by placement of a 0.018-inch wire into the vein. The venotomy site is preferably above the elbow and attention should be paid to the potential for the needle to pass through a nerve; if the patient complains of pain radiation down the extremity, another site should be selected. The skin site is then dilated with a 4- to 6-Fr dilator and a measurement wire placed to the inferior SVC and the length noted. The PICC is then cut to this length and inserted through a small peel-away sheath and secured to the skin. While extremity veins are usually selected for PICC placement, the lines can be placed in almost any venous structure that is:
Close to the skin.
Visible by US.
Is large enough to accept the catheter without it occluding.
The tip can be placed into a central vein.
For example, the inferior epigastric vein, which travels along the abdominal wall, can be utilized for access. Preservation of venous access sites is essential in the treatment of any patient as there are only a handful, the internal/external jugular, subclavian (not preferred), femoral, and directly into the IVC immediately superior to the pelvic brim. The latter is a last resort since it requires direct puncture into the IVC and tunneling through the soft tissues of the flank. The femoral venous access is also not preferred due to the difficulty in managing the sterility or cleanliness of the site. There are often more central line infections when the femoral vein is utilized.
A new variation of the PICC line is the tunneled PICC, which is inserted in the jugular vein and tunneled inferiorly similar to any other tunneled line. These lines are 6 Fr in diameter, double lumen, and placed centrally. These have been used more frequently in a patient who is not on dialysis currently but is expected to be placed on it imminently. This type of central line spares the veins of the extremities so that they can be used for dialysis shunts/grafts.
All tunneled lines have a Dacron cuff in the portion that is passed through the tunnel so that the skin can grow into it and secure it. This occurs 3 to 4 weeks after placement so that the skin sutures are not as important in these longer-term lines. This, however, affects their removal, as blunt dissection along the catheter track is necessary to easily remove them. Sometimes, the catheter is removed and leaves the Dacron cuff within the soft tissues. To remove it, it requires a cutdown and exposure with its removal. Usually these cuffs are left in place, but the patient must be told about it so that if they feel a small lump they know what it is. Mammography will detect these cuffs and, without the information and documentation that they are the residua of a tunneled line, may be mistaken for a malignancy.
Nontunneled central lines are placed in a similar fashion without the necessity of a tunnel. These are utilized when the patient is an in-patient and removed before their discharge. Adequate suturing is necessary to avoid displacement of the line.
Interventional Therapies
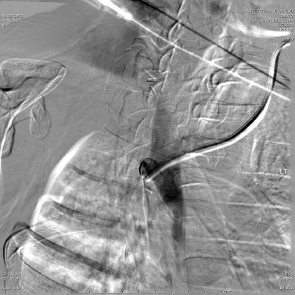
The first embolotherapy requests were likely for controlling bleeding from tumor invasion into adjacent structures. This requires superselective catheterization and led, eventually, to the development of the microcatheter and 0.018-inch and smaller diameter guide wires. Hypervascular tumors such as sarcomas, renal cell carcinomas (RCC), hepatomas, and melanomas invade adjacent organs such as the pancreas, kidney, or duodenum and cause hemorrhage that can be life-threatening. While coil embolization of the vessel causing the hemorrhage may or may not be sufficient to halt it, other embolic agents need to be considered and individualized for every patient. The choice of embolic agent is critical since the use of particulates to embolize bleeding should only be utilized when there are no normal tissues downstream to infarct. For example, the invasion into the duodenum of pancreatic carcinoma usually requires the placement of 5- to 6-mm-diameter platinum coils into the proximal and distal gastroduodenal artery (GDA). If particles are utilized, the pancreatic head will be embolized, resulting in at least pancreatitis, pseudocyst, or necrosis ( Fig. 4.9 ).
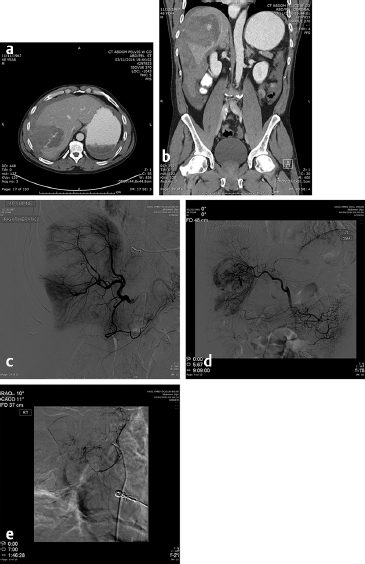
Embolotherapy
Patients most likely to benefit from directed therapy to treat hemorrhage from a tumor are those in whom the tumor mass can be separated on angiography from normal tissues. Embolization of normal tissues may be necessary to halt the bleeding, but it is important to recognize the collaterals that form to feed the normal embolized tissues. To perform embolotherapy correctly and safely, the choice of embolic agent is the first critical decision to be made after accepting the referral. This choice begins with the decision of whether they are to be permanent, semipermanent, or temporary. Permanent embolic agents include coils or vascular plugs made of nitinol. Each of these is available in a range of sizes that will allow most vessels to be embolized in a few minutes after placing the catheter. Placement of a microcatheter is easier than a 5-Fr catheter and the microcoils are effective since they are not only made of platinum, but also have Dacron fibers interwoven into them that promote platelet aggregation and thrombus formation. Other permanent agents include ethanol, Sotradecol (which contains ~45% ethanol), or glue. These are more liquid agents and must be treated with extreme care since they can penetrate deeply into the small arterioles and cause necrosis. Ethanol is immediately toxic to the endothelial lining of arteries, causing this lining to slough and exposing the media, which is thrombogenic. When using ethanol, complete tissue destruction downstream from the catheter tip is expected and argues for meticulous catheter placement. The amount of ethanol to use is estimated by seeing the penetration into the tissues by contrast. The use of a balloon occlusion catheter may be of assistance when using alcohol, but it should be well aspirated before the balloon is deflated so that the products of the embolization are not refluxed into a neighboring artery causing embolization to areas that are not intended. For example, ethanol has been utilized for the embolization of RCC prior to nephrectomy so that the devascularized tissues will not cause extraordinary blood loss due to the hypervascular nature of the RCC. The use of an occlusion balloon has been promoted thinking that the balloon will keep the debris and alcohol contained. However, if this catheter is not completely and well aspirated and flushed and re-aspirated after the ethanol use, some ethanol and debris can be, and has been reported to have, refluxed into the superior mesenteric artery. Others have foregone the use of the occlusion balloon arguing that the blood flow is necessary to carry the ethanol into the distal arterioles to have a more complete embolization.
Glue, another permanent agent, presents its own set of peculiarities, problems, and demands. Catheters must be flushed with D5W before injecting the glue through a glass syringe that is used to aspirate the glue from a glass container. The glue should not come into contact with plastic syringes or an ionic solution until it arrives in the blood stream as either causes it to begin polymerization. The glue itself is not radio-opaque but, when mixed with the enclosed tantalum powder, becomes easily visualized under fluoroscopy. When the flow is fast and distal embolization is desired, a more liquid and delayed polymerization is used by adding Pantopaque or Ethiodol to the mixture. If more proximal obstruction is desired, the less the polymerization is delayed. However, the sooner the polymerization begins, the more likely the catheter can be permanently glued in place if not removed as the last bit of glue is placed. There are catheters in the market that can retard the attachment of the glue so that the likelihood of being glued to the artery is now significantly less. Some of the gluelike agents will also not cling to the catheter as well as the NBCA (n-Butyl cyanoacrylate) glue. The permanent liquid agents are usually used in the setting of arteriovenous malformation treatment and less so in tumor or hemorrhage.
While many consider particles to be permanent, they are actually a semipermanent agent since, over time, they are incorporated into the vascular wall and flow may be re-established. This may occur over days to weeks so that the tumor distal to the embolization site may have become necrotic. The size of the particle is a key determinant to how fast the recanalization occurs. The larger the particle, the more proximal the occlusion, meaning collaterals are more likely to quickly form as these vessels are larger, more available, and more numerous. The smaller the particle, the more distal it occludes the artery, and necrosis of tissue may ensue. A general rule of thumb for selection of size of particle is that if the artery in which the catheter is placed feeds only tumor, it is safe to use the 40-µ-diameter particles, the smallest particles currently available. However, if this particle size is utilized and normal tissue is embolized, there will be normal tissue necrosis with islands of viable tumor present. If normal tissues must be sacrificed when embolizing, larger particles, 100 to 300 µ in diameter, can be used. Larger particles than these cause collateral formation almost instantaneously.
A relative contraindication to embolotherapy of tumor in the liver is occlusion of the portal vein. Conventional wisdom has it that, by occluding the hepatic artery, all flow to the liver will be lost and necrosis of the lobe will occur. This is rarely the case, as the intrahepatic arterial collaterals will open quickly and supply the embolized lobe as long as the smallest agents are not utilized. If a lobe of liver has been completely replaced by tumor, and, in cases of hepatocellular carcinoma (HCC), for example, the portal vein is invaded or occluded by tumor, there has been a fair amount of hesitation to using embolotherapy because of the risk of necrosing the entire lobe. However, if the tumor has completely or nearly completely replaced the lobe, the only tissue that will necrose is tumor. In such a case, the smallest embolic agent available, 40-µ-diameter particles, can be used to insure that the tumor is thoroughly treated.
Chemoembolization has changed from its original design of a slurry of Ethiodol or lipiodol, both iodinated poppy seed oils, with a chemotherapy agent. The oil is phagocytized into the cancer cell bringing the emulsified chemotherapy agent with it as was demonstrated by making autoradiographs of 131I-lipiodol. 9 The chemotherapy agents utilized in these treatments include Adriamycin, cisplatin, mitomycin C, or irinotecan, individually or in combination. While such “traditional” chemoembolization showed promise as a treatment for metastatic tumor in the liver, it was especially operator dependent to insure the optimal chemotherapeutic concentration and emulsification since the lipiodol actually dissolved the polycarbonate three-way stopcocks that were used to emulsify the oil and chemotherapy agent. Additionally, the commercial availability of the lipiodol/Ethiodol has been sporadic. After the emulsion had been administered, the hepatic arteries were embolized with particles until stasis of flow occurred. The exact definition of “stasis” is problematical since this is another operator-specific variable.
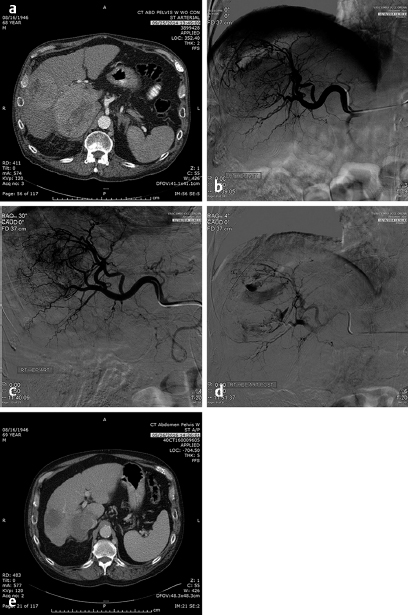
These problems have been somewhat circumvented by the use of drug-eluting beads that are soaked in either Adriamycin or irinotecan for a specified period to obtain maximal concentration and then the tumor is embolized with a specific dose of the bead–chemotherapy combination and may or may not be embolized to stasis after the delivery of the chemotherapy-loaded beads. Unlike the original chemoembolization, the cells are bathed in the chemotherapy until the dose has become depleted without a mechanism to insure that the chemotherapy will actually get into the cell itself. Additional problems with this type of chemotherapy include the use of irinotecan, a mildly effective chemotherapy agent on its own, but when used systemically, the effectiveness is related to the conversion of irinotecan to SN-38 by an enzyme occurring in normal liver but not in tumors. SN-38 is 1,000 times as effective as the irinotecan. Because of the sclerotic nature of irinotecan, patients report significant pain, above and beyond the postembolization pain. The exposure of the tumors to a high dose of either irinotecan or Adriamycin for a few hours when neither are very effective chemotherapy agents in the tumors in which they are used systemically (irinotecan – 15% in metastatic colorectal cancer and Adriamycin – 18% in HCC when used as single agents) has led to questioning of the rationale of this type of chemoembolization. Potentially, the chemotherapeutic agents are merely sclerotic agents and extend the range of the embolotherapy to smaller vessels. Research is beginning to determine the mechanism of action of the chemotherapy drug used in the drug-eluting beads. Nevertheless, response to this therapy has been reported to be in the range of 50% in HCC and 40% in metastatic colorectal cancer to the liver, which is not significantly different from bland embolization. 10 Bland embolization of tumors has also been utilized to enhance the effect of RFA treatment of liver and renal tumors 11 ( Fig. 4.10 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



