Hematopoietic Cell Transplantation in Hodgkin Lymphoma
Weill Cornell Medical College, New York Presbyterian Hospital, New York, NY, USA
Autologous transplantation is the standard treatment for patients with recurrent Hodgkin lymphoma (HL). It is an overall well-tolerated treatment that can result in durable remissions, and it cures a large proportion of patients with chemosensitive disease and some with chemotherapy-refractory disease. Its role has been extensively reviewed. Here, we address some management questions that remain or that have arisen as a consequence of novel developments in the field.
1. Does residual marrow involvement affect the results of autologous transplantation?
There is considerable evidence that occult marrow or stem cell involvement as determined by a variety of methods (polymerase chain reaction, clonogenic assays, etc.) contributes to disease recurrence in patients with lymphoma undergoing autologous transplantation. Further evidence of the detrimental effect of occult marrow involvement comes from a comparative study showing that patients with lymphoma undergoing syngeneic transplantations have a lower risk of disease recurrence than those undergoing autologous transplantation. Such studies provide compelling evidence that submicroscopic marrow or stem cell involvement is common in lymphoid malignancies and constitutes an adverse prognostic feature. Whether circulating clonogenic lymphoma cells exist or can be mobilized in HL remains to be determined. Tantalizing but preliminary data suggest that they might.
Only a minority of patients with HL present with overt marrow involvement at the time of initial diagnosis or at the time of disease recurrence, and such patients pose a peculiar challenge. In an apparent paradox, such overt marrow involvement does not constitute an absolute contraindication for autologous transplantation. Several cases have been reported of patients with overt marrow involvement who underwent bone marrow harvest followed by autologous transplantation and who achieved durable complete remissions.
Cells from HL collected during marrow harvest therefore do not always lead to disease recurrence, and marrow involvement should thus be considered in the context of the overall patient assessment and only as a relative contraindication. By itself, it is not sufficient reason to recommend a change in treatment strategy or to recommend an allogeneic transplant with its attendant risk for serious complications. The likelihood of durable response after autologous transplantation should take into account other, better-established risk factors such as disease response assessed by radiological or functional testing.
2. What is the best conditioning regimen?
Early studies of autologous transplants for Hodgkin lymphoma used total body irradiation (TBI)-based regimens, which were also widely used at the time in allogeneic transplantation. TBI-based regimens have not, however, been shown to be superior to chemotherapy-based regimens; are logistically more difficult to organize; cannot be utilized in patients with prior dose-limiting radiation; and probably are associated with higher rates of late therapy-related acute myeloid leukemia and myelodysplastic syndrome (t-AML–MDS). Most centers have therefore abandoned their routine use in HL.
The most commonly used chemotherapy-based regimens include high doses of the nitrosourea BCNU (bis-chloroethylnitrosourea) combined with another alkylating agent such as cyclophosphamide or melphalan, and it often also incorporates etoposide and sometimes high-dose cytarabine [e.g., carmustine, etoposide, cytarabine, and melphalan (BEAM); carmustine, etoposide, cytarabine, and cyclophosphamide (BEAC); and cyclophosphamide, carmustine, and etoposide (CBV)]. The reported treatment-related mortalities associated with the use of these regimens have declined over the years thanks to improvements in stem cell support and infection prophylaxis and treatment. Toxicity also depends somewhat on the doses of BCNU utilized, which range from 300 mg/m2 to 800 mg/m2. At the higher end of this dose range, there is a considerable incidence of pulmonary toxicity and also of sinusoidal obstructive syndrome (also known as veno-occlusive disease). Doses of BCNU exceeding 450 mg/m2 should be avoided.
Regimens incorporating busulfan instead of BCNU are also increasingly being used and may have less risk for pulmonary toxicity. Other regimens incorporating active agents such as gemcitabine, bendamustine, and thiotepa have been developed in attempts to improve efficacy. Early results are promising but ideally should be compared in a randomized trial against older regimens.
3. What is the role of positron emission tomography (PET) scanning before transplantation?
Patients with HL who have received curative therapy often remain with enlarged fibrotic masses. Functional imaging is necessary to assess response and prognosis. Older methods based on gallium scanning have been superseded by PET, which is less time consuming, has better resolution, and can be combined with computed tomography (CT) scanning. In a retrospective study by Jabbour et al. (2007), functional imaging of Hodgkin patients before transplantation using both PET scans and gallium scans was superior to radiologic imaging alone in predicting both relapse and overall survival (OS) post-transplantation.26 Patients with a positive functional image before transplantation had progression-free survival (PFS) of 23% and an OS of 58%, compared with 69% and 87%, respectively, in those with negative functional imaging. Similarly, patients in partial remission with a positive functional image had a 3-year PFS of only 27% versus 51% if the functional imaging was negative. In a multivariate model, positive functional imaging was found to be independently prognostic of PFS. Several other studies also suggested that a pretransplantation positive PET in HL confers a worse prognosis. And a meta-analysis of 16 studies confirmed the prognostic value of a pretransplantation PET scan in predicting both relapse and OS in patients with lymphoid malignancies.
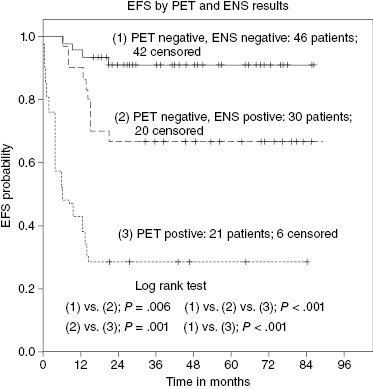
But the interpretation of PET scan results has its own challenges with ongoing debates over threshold values and ever-changing technologies. It is not uncommon to encounter false-positive PET scan results due to a variety of causes (brown fat, thymic rebound, bleomycin-induced inflammation, etc.). Sometimes, biopsy is required. But even in cases where there is consensus that PET positivity represents residual disease, this does not constitute an absolute contraindication for transplantation. For example, in a study from the University of Washington, patients with positive PET scans prior to transplantation had a 3-year expected event-free survival (EFS) of 42%, which was considerably worse than that of PET-negative patients but still respectable. Other prognostic factors may aid in assessing prognosis in such patients and to identify those with a dismal prognosis. A prospective study sheds some light on this complex quandary. It confirmed that patients with a negative PET had an EFS of >80% versus only 28.6% in those patients whose PET was still positive pretransplantation. But in a multivariate analysis, PET scan and extranodal disease were both significant such that patients with a negative PET scan but a history of extranodal disease truly had a dismal prognosis (Figure 37.1).
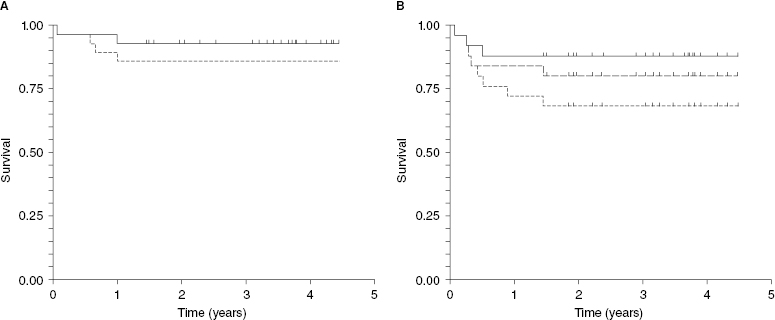
Recently, a British group developed an algorithm to utilize PET scan in treatment assignments to autologous versus allogeneic transplantation. Patients who are PET negative after salvage therapy undergo autologous transplants, but those with positive scans are referred for allogeneic transplants. They report approximately 90% long-term survival for those undergoing autologous transplantation. Of interest, long-term survival for those undergoing allogeneic transplantation, all of whom had positive PET prior to transplantation, is also in the 90% range. Only two of 25 patients died of treatment-related causes after allogeneic transplantation. This low treatment-related mortality can be explained best by the relatively young age of the patients, their rather limited exposure to prior chemotherapies, and consequently their good performance score and limited comorbidities. Allogeneic transplantation was quite effective at controlling Hodgkin lymphoma. Although some patients relapsed after allogeneic transplantation, many responded to immune manipulations such as donor lymphocyte infusion and/or withdrawal of immune suppression (Figure 37.2).
4. What is the role of autologous transplantation in the era of escalated BEACOPP?







