3 Essentials of Radiation Oncology
Introduction to Radiation Oncology
Radiation oncology is the medical use of ionizing radiation in cancer treatment to control malignant cells. Ionizing radiation damages the DNA (deoxyribonucleic acid) of cells, either directly or indirectly, by forming free radicals and reactive oxygen species. Whereas healthy normal cells are differentiated and can repair themselves, in cancer cells DNA damage is inherited through cell division and the accumulation of this damage to malignant cells results in cell death. Ionizing radiation can be produced by electron, photon, proton, neutron, or ion beams. Provided below is a short summary of the establishment of radiation oncology, the overall structure of its governing bodies, a definition of the scope of practice of radiation oncology, and the primary treatment approaches that are employed.
Brief History of the Specialty
The field of radiation therapy (RT; also known as radiotherapy and radiation oncology) arose shortly after Wilhelm Rontgen discovered X-rays in 1895. The use of X-rays was rapidly taken up by physicians for such purposes as diagnosing broken bones and locating foreign objects in patients. However, in 1896, Antoine-Henri Becquerel recognized that certain elements spontaneously emitted rays or subatomic particles from matter; this property came to be known as radioactivity. In their experiments, which led to the identification of polonium and radium, Pierre and Marie Curie observed radium destroying diseased cells. This was the first indication that radiation was a boon not only to disease diagnosis, but also to treatment.
In the early 1900s, the field of RT expanded quickly, spawning a new era in medical treatment and research. Physicians initially tested exposure to X-rays and based their clinical practice on their observations. Although the mechanism of action for radiation effects had not yet been identified, reports of tumor control or regression began to fund the scientific literature. Researchers were optimistic about the benefits of radiation, but wary of its possible harms.
Roentgenology, as it was then known, assumed a role in World War I, wherein radiologic equipment was used in field hospitals, and French and American soldiers were trained to take X-rays; thus, the demand for radiologic service and technology continued to rise. In time, many physicians purchased X-ray machines for their office practices, and several even announced their specialty as radiology. Between the world wars, physicists and biologists continued to discover the mechanisms of radiation and how to measure the dosages accurately. Higher energy X-ray machines and new radium devices came on the market.
Skip to the 1960s, when megavoltage treatment machines, known as linear accelerators or Linacs, were introduced. Linacs could produce uniform doses of high-energy beams, allowing the penetration treatment of tumors deep inside the body, yet sparing excessive damage to overlying skin and other normal tissues. In the 1970s and 1980s, computers could be used to plan treatment. The advent of new imaging technologies, including magnetic resonance imaging (MRI) and position emission tomography (PET), moved RT from 3D conformal to intensity-modulated radiation therapy (IMRT) and image-guided radiation therapy (IGRT). IMRT with different intensities of radiation beams allows the simultaneous delivery of different doses of radiation. Higher doses of radiation can be delivered to tumors and lower doses to nearby healthy tissue. A distinct benefit of IGRT is in allowing the radiation oncologist to image the tumor immediately before and during the treatment, allowing clinicians to better see and target tumors. This has resulted in better treatment outcomes, more organ preservation, and fewer side effects.
Governing Bodies of Radiation Oncology
In the United States, the field of radiation oncology is governed by two primary groups: the American College of Radiology (ACR) and the American Society for Therapeutic Radiology and Oncology (ASTRO). ACR, with more than 30,000 members, is the principal organization of radiologists, radiation oncologists, and clinical medical physicists. The college is a nonprofit professional society whose primary purposes are to advance the science of radiology, improve radiologic services to the patient, study the socioeconomic aspects of the practice of radiology, and encourage continuing education for radiologists, radiation oncologists, medical physicists, and persons practicing in allied professional fields.
ASTRO became its own entity, endeavoring to, among other efforts, assist with continuing medical educational opportunities and standards for educating RT trainees. In the 1990s, the society began to communicate the specialty’s goals and achievements to the general public, compose strategic 5-year plans to achieve its goals, represent member interests in legislative and regulatory spheres, and foster leadership growth within its ranks. In 1998, ASTRO became its own independently managed society with its own headquarters in Fairfax, VA. ASTRO then increased involvement in socioeconomic issues and expanded efforts in outcomes research. In the new millennium, ASTRO has ingrown its efforts in health policy and government relations, as well as Medicare and Medicaid issues. ASTRO continues to focus on issues of importance to its members, such as technology advances, treatment regimens, communications dissemination initiatives, and newer issues such as the importance of radiobiology. ASTRO also continues to work with other medical organizations and specialties to share research and information. In 2008, the society changed its name to the American Society for Radiation Oncology, still retaining the acronym ASTRO.
ACR periodically defines new practice parameters and technical standards for radiologic practice to help advance the science of radiology and to improve the quality of service. ACR and ASTRO collaborate on scope of practice and practice parameters.
Definition of Radiation Oncology Scope of Practice
Radiation oncology is one of the primary disciplines, along with surgical and medical oncology, involved in cancer treatment. RT is a cornerstone of cancer care and is one of the most powerful therapies in oncologic practice, with the capability of preserving organ and function while providing the potential for cure. RT is the use of ionizing radiation to destroy or inhibit the growth of malignant tissues. It is also used in selected clinical situations to inhibit the growth or modulate the function of tissues in certain benign diseases. Separate practice parameters and standards define the appropriate use of external beam therapy, brachytherapy, and other therapies using radionuclides. RT with either curative or palliative intent is used to treat up to 60% of all patients with cancer. 1
In the current economic environment, the widespread use of new and more expensive technologies has reduced the cost of RT relative to that of systemic therapies. Finding and then justifying its appropriate use using the tools of comparative effectiveness have become a major and urgent responsibility for investigators. 2
Early in 2011 ASTRO’s Board of Directors began formally discussing the future of the basic sciences of radiation oncology. Its focus was the current state and potential future direction of basic research within the specialty. In August 2011, a Cancer Biology/Radiation Biology Task Force (TF) was initiated and charged with developing an accurate snapshot of the current state of basic (preclinical) research in radiation oncology, its relevance to modern radiation oncology clinical practice, and the education of trainees and attending physicians in the biological sciences. The TF was further charged with making suggestions as to critical areas of biological basic research investigation that might be most likely to maintain and build further the scientific foundation and vitality of radiation oncology as an independent and vibrant medical specialty. 3
Main Radiation Oncology Treatment Approaches
Radiation used in the treatment of malignant cells can be directed into the body from either an internal or external source. In the past two decades, technologic advancements in radiation oncology and diagnostic radiology have allowed for innovative approaches to therapy. The two main available approaches are external beam radiation therapy (EBRT) and brachytherapy. A radiation oncologist may have at his/her disposal external beam treatment equipment that provides beams other than conventional photon and electron beams (e.g., proton beams). Special expertise on the part of the radiation oncologist as well as the physics and therapy staff is required for safely using this treatment equipment. Brachytherapy (radioembolization [RE]) may be used for many sites and may be delivered with either low-dose-rate or high-dose-rate techniques.
Powerful technology growth in the modern era, such as computer hardware upgrades, including megavoltage linear accelerators, and software programs that enable conversion of computed tomography (CT) or MRI datasets into three-dimensional (3D) virtual patients have meant expanding effects in radiation oncology therapies. With accurate 3D models of the patient to work from and estimates in real time of radiation dose deposition within the patient, radiation oncologists can attempt to deliver the higher doses of radiation that have a chance to control tumors while sparing the nonmalignant cells. Technologic advances in EBRT and brachytherapy as well as the commercial availability of radioactive microsphere products allow a broader spectrum of treatment to those with primary and metastatic disease ( Fig. 3.1 ; Fig. 3.2 ).
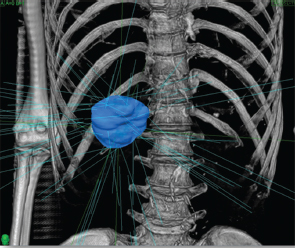
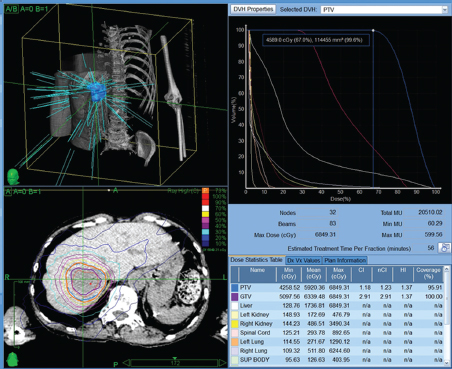
Continuous low-dose radiotherapy is the type of brachytherapy delivered by yttrium-90 (90Y) microspheres. Compared with external beam (via modern linear accelerators), which is high dose (500 cGy/ min), pulsatile (once/24 h), and delivered 5 d/wk, RE delivers effective dose rate radiation at low dose (50 cGy/min), continuously (every second), and 7 d/wk for 14 days.
Physics of Radiation Oncology
The seminal event in the tumor cell targeted by radiation is ionization of the DNA located in the nucleus. Although many potential targets in the malignant cell can produce radiation damage, the cessation of reproductive capacity is the true goal of RT. The direct effect on DNA—the photon or β particle striking one of the two DNA strands—causes irreversible damage and occurs in just 25% of lethal interactions. The indirect radiation effect on DNA, which leads to cell death in 75% of encounters, involves a water molecule–absorbing radiation energy, ejection of a Compton electron from the outer shell of the oxygen atom, and the creation of a free radical. The highly unstable and reactive free radical develops within 4 nm of the DNA strand, which leads to either a single-strand or a double-strand DNA break. If unrepaired, the cell will lose reproductive integrity, and die either via apoptosis or after a few additional cell divisions. The presence of oxygen is critical for the successful creation of free radical damage near to the DNA. 4
Radiation that is of sufficient energy to cause ionization of cellular contents is used therapeutically and is either an electromagnetic or a particulate energy form. Electromagnetic energy, or photons, can be produced naturally by decay of radioactive isotopes (γ-rays) or by an electrical device accelerating electrons, which abruptly stop in a target, releasing energy (X-rays). Particulate energy most commonly used for cancer therapy is electrons (charge: −1; energy: 0.511 MeV [million electron volts]; mass: 9.109382910−31 kg), but others in limited use include protons (charge: +1; energy: 938.27231 MeV; mass: 1.67262310−27 kg, which is ~2,000 × electron), a-particles (helium ions), and neutrons (same mass as proton, no charge).
External Beam Radiation Therapy
External beam RT delivers radiation outside the body; EBRT that employs X-rays is the most commonly used method for treating nearly all cancers. Photons, which are discrete packets of electromagnetic energy, cause cell damage or cell death via apoptosis, colliding with a cell and transferring a portion of energy to the cell. This interaction exchanges some energy to the cell, and the photon itself is deflected with a reduction in its energy. The energy absorbed by the cell will possibly create damage to the DNA, leading to cell death.
In the 1960s through early 1980s, external beam radiation was, in fact, the delivery of photons from radioactive decay of 60Co. Although it yielded photon energies with sufficient penetrating power for most tumors, it could not be used for deep abdominal or pelvic tumors without delivering a much higher dose more superficially in normal tissues. In addition, the physical radiation beam itself possessed a relatively wide beam edge or penumbra, which made precise targeting impossible even at shallow depths of tissue.
Over the past 20 years, linear accelerators have replaced 60Co machines virtually everywhere, generating photons by accelerating electrons approximately at the speed of light prior to striking a target. This activity converts kinetic energy and mass into electromagnetic energy photons. Linear accelerators generate photons of much higher energy than 60Co and thus are able to reach any deep tumor in the body of most patients, without creating excessive hot spots or resulting in doses higher than that of the tumor along the photon path in the body. In absolute numbers, 60Cot can deliver γ-rays (photons) of two energies, 1.17 MeV and 1.33 MeV; although some accelerators are capable of maximum photon energies of between 4 and 25 MeV, most centers use 6 to 18 MeV. Linear accelerators also can produce electron beams, which differ from photon beams in that electrons are particles with mass and charge, and thus have a finite range of tissue penetrance, allowing for treatment of more superficial tumors, while significantly sparing deeper normal tissues.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



