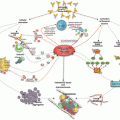
Clinical manifestation
%
Organ involved
Kidney
73
Renal failure
77
Proteinuria
29
Arterial hypertension
24
Hematuria
16
Lung
60
Acute respiratory distress syndrome
36
Pulmonary embolism
26
Alveolar hemorrhage
12
Pulmonary edema
8
Brain
56
Stoke
40
Encephalopathy
39
Seizures
15
Headache
8
Heart
50
Heart failure
44
Myocardial infarction
30
Valvulopathy
28
Libman-Sacks endocarditis
13
Skin
47
Livedo reticularis
43
Cutaneous necrosis
26
Cutaneous ulcers
24
Purpura
14
Liver
39
Elevated liver enzymes
63
Hepatomegaly
10
Liver failure
9
Jaundice
7
Peripheral vessel
37
Peripheral venous thrombosis
69
Peripheral arterial thrombosis
46
Gastrointestinal
24
Gastrointestinal bleeding
18
Ileus
4
Spleen
18
Adrenal glands
10
Thrombocytopenia is the most common laboratory feature (67%). Hemolysis is also frequently reported (37%) and is usually associated with schistocytes (22%). Some patients developed DIC (11%). Among CAPS patients, most had circulating lupus anticoagulant (LA) (83%), IgG anticardiolipin antibodies (aCL) (81%), and/or IgG aβ2GPI (78%), while IgM aCL (49%) and IgM aβ2GPI (40%) were less often found. Antinuclear antibodies were detected in 57% of cases and anti-double-stranded DNA antibodies in 32%, figures explained by clinical features of SLE in up to 40% of cases. Antibodies against the extractable nuclear antigens were less often detected: anti-RNP in 8%, anti-Sm in 5%, anti-La in 4%, and anti-Ro in 9% (percentages calculated according to the number of patients with these tests recorded).
“Thrombotic Storm ” and Differential Diagnosis of Catastrophic Antiphospholipid Syndrome
Thrombotic storm refers to a clinical presentation of acute development of multiple thromboembolic events involving diverse vascular beds [18]. Affected individuals are typically young, and thromboembolic events often involve unusual sites [18, 19]. The term “thrombotic storm” was first used by Kitchens in 1998, and then the clinical phenotype was proposed by a multidisciplinary group for clinical studies. The characteristics of thrombotic storm include (a) an underlying hypercoagulable state; (b) a provocative factor or “trigger” associated with initiation; (c) new thromboembolic events developing rapidly, especially if therapy is delayed; (d) importance of prompt initiation of antithrombotic therapy ; and (e) good long-term prognosis, if the cycle of thrombosis is interrupted early. Of the six patients in Kitchen’s report, three likely had CAPS [20].
In addition to CAPS, several disorders are included under the umbrella of thrombotic storm including [21] atypical presentations of thrombotic thrombocytopenic purpura (TTP), in which thrombotic occlusions may develop days to weeks before the development of thrombocytopenia and characteristic microangiopathic changes [22, 23]. Catastrophic thrombosis occurs in patients with cancer; the combination of CAPS and malignancy has been described in several patients. Recurrent thromboembolic events and antibodies against platelet factor 4/heparin complexes have been described, despite no recent exposure to heparin, an entity referred to as spontaneous heparin-induced thrombocytopenia (HIT) [24]. A small subset of patients with thrombotic storm have no recognized associated hypercoagulable states, a condition referred to as “idiopathic” thrombotic storm [21].
The initial diagnostic evaluation of a patient presenting with multiple thrombotic events includes imaging studies to define the location and extent of thrombotic occlusions, which may guide subsequent targeted therapeutic interventions and laboratory studies that include a complete blood count and blood film, screening coagulation studies (prothrombin time, activated partial thromboplastin time [aPTT], and fibrinogen), a metabolic profile, and testing for aPL. For patients in whom the initial assessment raises concern for occult malignancy, additional diagnostic imaging and/or laboratory studies should be obtained to confirm the diagnosis [25].
Therapeutic anticoagulation must be initiated promptly, as new thromboses occur rapidly. Unfractionated heparin is an effective anticoagulant in this setting, given the ability to titrate dose, familiarity with management in the periprocedural setting, and availability of a reversal agent (which should be reserved for cases of disastrous hemorrhagic complications only). A parenteral direct thrombin inhibitor, such as argatroban and bivalirudin, can be used if there has been relatively recent exposure to heparin and delayed HIT is in the differential diagnosis. Low molecular weight heparin is effective in the majority of patients with multiple thromboses and malignancy, assuming normal renal function.
Many patients will have thrombocytopenia on presentation. If review of the peripheral blood film by the hematologist reveals schistocytes, the patient most likely has a thrombotic microangiopathy, such as TTP or CAPS. A prolonged aPTT may suggest the presence of an aPL, but this needs to be further evaluated with additional laboratory testing. If either diagnosis is suspected, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13) level should be sent and plasma exchange should be initiated; a diagnosis of TTP is supported by a markedly decreased ADAMTS13 level (e.g., <5%). Corticosteroids are also commonly administered to patients with CAPS [17] as well as acquired (autoantibody mediated) TTP [26].
Patients with a new or unexpected thrombocytopenia on presentation in the absence of a microangiopathic hemolytic anemia, or who rapidly exhibit a drop in the platelet count after heparin is initiated, may have an unusual presentation of HIT, referred to as spontaneous HIT [24]. Laboratory testing for anti-platelet factor 4/heparin antibodies should be sent, and the patient should be switched to an alternative anticoagulant, either a parenteral direct thrombin inhibitor or fondaparinux .
If the prothrombin time (PT) is also prolonged, and/or the fibrinogen level is decreased, the possibility of a consumptive process, such as DIC, needs to be considered. Depending on the severity of the coagulopathy, DIC can complicate safe administration of anticoagulant therapy [21]. Another cause of an elevated PT is antibody to prothrombin, which sometimes accompanies aPL, whether or not the patient has CAPS. If this antibody is the cause, corticosteroid therapy is indicated.
Management of Catastrophic Antiphospholipid Syndrome
The current recommendations for the treatment of CAPS are based on the following steps:
- A.
- B.
Decide if the patient needs supportive measures, such as hemodialysis, mechanical ventilation for respiratory failure, or inotropic drugs [29].
- C.
Combine anticoagulation (full-dose unfractionated or low molecular weight heparin) with glucocorticoids (GC, intravenous pulses [500–1000 mg/day of methylprednisolone or equivalent for 1–3 days] or oral or intravenous doses of 1–2 mg/kg/day of prednisone or methylprednisolone or equivalent) [30]. If the clinical course is satisfactory, maintain heparin for at least 7–10 days; after which consider substituting with oral anticoagulation (warfarin or other vitamin K antagonists; the use of the direct-acting oral anticoagulants cannot be recommended until there is better information regarding their efficacy and safety in this condition). If the baseline aPTT is prolonged, adequacy of heparin anticoagulation should be confirmed with an anti-factor Xa assay (targeting the upper end of the recommended heparin level of 0.3–0.7 anti-factor Xa units/mL).
- D.
Consider plasma exchange (PE) and/or intravenous immunoglobulin (IVIG) in cases of life-threatening situations or signs of microangiopathic hemolytic anemia [14, 30, 31]. (We use 5% albumin solution as a choice replacement fluid for PE.) There are no dosing recommendations on the dose of IVIG; suggested doses range from 400 mg/kg once at the low end up to 400 mg/kg daily for 5 days [total dose of 2 g/kg] at the high end, sometimes continued monthly. Many clinics administer IVIG together with plasma exchange, spacing interventions to allow IVIG to have its effect before removing it [32].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree