Case study 129.2
A 56-year-old woman who previously had a partial nephrectomy for renal cell cancer is found to have an enlarged retroperitoneal lymph node on CT scan performed in the emergency room after a fall at home. She is seen by medical oncology for a recommendation for treatment. She has no other medical issues of which she is aware, and she is completely asymptomatic. On imaging, she has a single retroperitoneal lymph node that is 2.7 cm in maximum dimension. A CT-guided biopsy confirms the presence of metastatic renal cell carcinoma. She would like your input on data she heard on the news about anti-PD-L1 and anti-PD-1 monoclonal antibodies.
1. Which of the following is true about these agents?
- They bind to a target on a tumor and directly destroy the tumor
- There is a defined test to help determine the likelihood of benefit
- These agents indirectly assist in tumor destruction
- Side effects of these drugs occur often but are clinically insignificant
The accepted mechanism of action of checkpoint inhibitors, which include anti-PD-L1, anti-PD-1, and anti-CTLA4 monoclonal antibodies, is not a direct effect on tumors. Although some antibodies targeting PDL-1 may induce antibody-dependent cellular cytotoxicity (ADCC), this requires other immune cells to become involved to kill the tumor, and thus answer A is incorrect. Indeed, these agents manipulate T-cell-signaling pathways that are part of the normal process to prevent autoimmunity or overstimulation of T-cells. A normal T-cell expresses both CD28 and CTLA4. When an antigen-presenting cell presents an antigen on MHC class I, it also must send a costimulatory signal to the T-cell through CD80 or CD86 (also called B71 and B72). That costimulatory signal is also called “signal 2” and is required for potent T-cell activation. However, upon T-cell activation, T-cells upregulate the regulatory receptor CTLA4 in response. This balance helps to prevent overstimulation of the T-cell, modulates T-cell-mediated lysis, and appears to play a significant role in preventing autoimmune diseases. Similarly, T-cells increase expression of PD-1 after signal 1 and 2 have provided the full “activation” signal. When PD-1 binds with its ligand, PD-L1 (B7-H1) or PD-L2 (B7-DC), a regulatory signal is sent to the T-cell, diminishing its activity. So, the mechanism of action of all of these agents is related to the prevention or reversal of T-cell inhibition by these signaling pathways. Unfortunately, there is not yet a validated test to indicate which patients’ tumors will respond to these therapies (answer B). It has been postulated that tumor cells overexpressing the ligands (e.g., PD-L1) may be more likely to respond, but this is not yet confirmed. In theory, blockade of PD-L1 may have less induction of autoimmune toxicity because the binding site is more likely to be on the tumor cells than elsewhere in contrast to CTLA4 or PD-1, both of which are expressed on T-cells, making their response to inhibition potentially less specific for tumor cells. In fact, the clinical studies of anti-CTLA4 (ipilimumab and tremelimumab) have reported significant autoimmune colitis, rash, and endocrine dysfunction. However, the phase I studies of anti-PD-1 and anti-PD-L1 appeared to have an improved toxicity profile, with fewer grade 3 or 4 adverse events. Notably, severe colitis was infrequently noted in the anti-PD-L1 study. As a result, answer D is not correct, these side effects are not common, but when they do occur, they can be severe and life-threatening. Figure 129.1 illustrates the interaction of these potential targets with T-cells, antigen-presenting cells, and tumor cells.
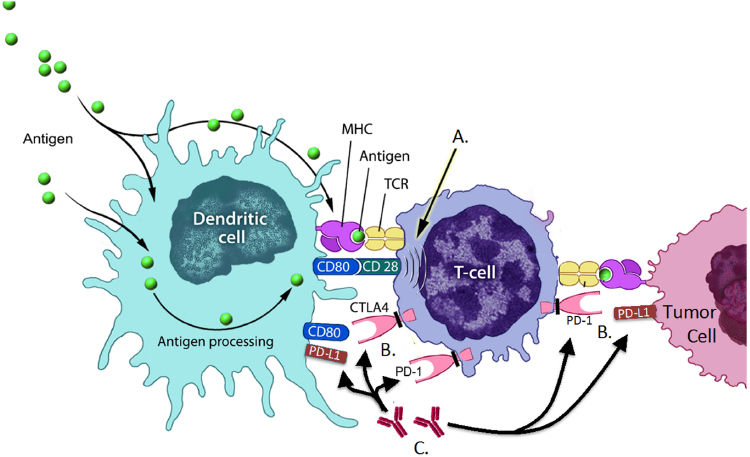 Figure 129.1 (A) T-cells become activated through a two-signal process. Signal 1 occurs when an antigen-presenting cell, in this case a dendritic cell (DC), presents an antigen via MHC class 1, which binds to the T-cell receptor (TCR). Signal 2 is the costimulatory signal, in this example CD80 on the DC binds to CD28 on the T-cell, activating the T-cell against the antigen presented. (B) After activation, the T-cell effector function can be downregulated or suppressed. Upon activation, the T-cell will increase the expression of cytotoxic T-lymphocyte antigen-4 (CTLA4) and programmed cell death receptor 1 (PD-1). When these receptors on the T-cell bind with their respective ligands (CTLA4 → CD80 and PD-1 → PD-L1), the effector function of activated T-cells is suppressed. (C) A therapeutic intervention that may allow T-cells to remain highly activated or become reactivated is inhibition of the regulatory signals provided by CTLA4 → CD80 and PD-1 → PD-L1 interactions. There are currently monoclonal antibodies in development or already approved targeting CDLA4, PD-1, and PD-L1. By binding these receptors and preventing the suppressive signal, T-cells are more capable of tumor lysis resulting in potential clinical benefit (Source: Adapted from Tarassoff CP et al. The Oncologist 2006;11(5):451–62. Reproduced with permission of AlphaMed Press).
Figure 129.1 (A) T-cells become activated through a two-signal process. Signal 1 occurs when an antigen-presenting cell, in this case a dendritic cell (DC), presents an antigen via MHC class 1, which binds to the T-cell receptor (TCR). Signal 2 is the costimulatory signal, in this example CD80 on the DC binds to CD28 on the T-cell, activating the T-cell against the antigen presented. (B) After activation, the T-cell effector function can be downregulated or suppressed. Upon activation, the T-cell will increase the expression of cytotoxic T-lymphocyte antigen-4 (CTLA4) and programmed cell death receptor 1 (PD-1). When these receptors on the T-cell bind with their respective ligands (CTLA4 → CD80 and PD-1 → PD-L1), the effector function of activated T-cells is suppressed. (C) A therapeutic intervention that may allow T-cells to remain highly activated or become reactivated is inhibition of the regulatory signals provided by CTLA4 → CD80 and PD-1 → PD-L1 interactions. There are currently monoclonal antibodies in development or already approved targeting CDLA4, PD-1, and PD-L1. By binding these receptors and preventing the suppressive signal, T-cells are more capable of tumor lysis resulting in potential clinical benefit (Source: Adapted from Tarassoff CP et al. The Oncologist 2006;11(5):451–62. Reproduced with permission of AlphaMed Press).
< div class='tao-gold-member'>



