Radiotherapy for Early-Stage Invasive Breast Cancer
The Ohio State University Comprehensive Cancer Center–Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, OH, USA
1. What is the appropriate method for postlumpectomy breast radiotherapy?
It is well acknowledged that breast conservation with lumpectomy and radiotherapy yields local control and overall survival that is equivalent to mastectomy in appropriately selected women. For over two decades, there was a nearly uniform approach to breast radiotherapy delivered post lumpectomy for breast conservation. Typical radiotherapy delivered approximately 50 Gy in 25–28 treatments (fractions) of 1.8–2.0 Gy each to the entire breast and in many cases was followed by additional or “boost” irradiation of 10–16 Gy in 5–8 fractions of 2 Gy each concentrated on the breast tissue immediately adjacent to the surgical cavity, for a cumulative total of 60–66 Gy. This meant nearly every patient received a total of 30–34 treatments over 6–7 weeks. This approach to breast radiotherapy has a long track record of success, yielding excellent in-breast cancer control, low overall toxicity rates, and high rates of acceptable cosmetic appearance of the breast. This conventional method of breast radiotherapy is still commonly recommended and in fact is an appropriate approach for this patient. However, more recent developments in our understanding of breast cancer failure patterns, radiobiology, radiotherapy delivery principles, and technology have allowed the development of alternative methods of postlumpectomy breast radiotherapy for which this patient is also a good candidate.
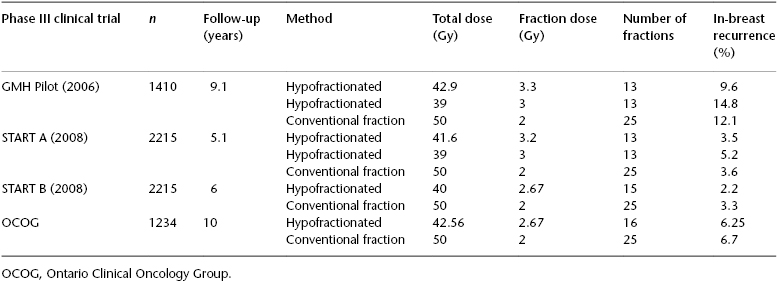
Hypofractionated whole-breast irradiation (HWBI) shortens the treatment course by the delivery of larger daily radiation doses of 2.67 Gy to a total of 40 or 42.67 Gy with 15 or 16 treatments over approximately 3 weeks’ duration. The radiation effect in tumor and normal tissues has both a linear (α) and quadratic (β) component in response, and the ratio of these two components is unique to specific normal tissue and tumors. Initial models examining the ratio of α and β influences on radiation response for breast cancer supported standard radiation fractionation of 1.8 to 2.0 Gy per day. Newer research and clinical trials have now established a model of α–β that supports altered fractionation to the entire breast of up to 3–4 Gy daily to yield comparable outcomes. Four randomized clinical trials have demonstrated that the in-breast local control and overall survival with HWBI are not inferior to those of conventional whole-breast irradiation (WBI) (Table 119.1). In 2010, the American Society of Radiation Oncologists (ASTRO) generated an evidenced-based clinical guideline supporting the use of HWBI as appropriate for postlumpectomy treatment in patients older than 50 years of age with pathologic stage T1–T2, N0 breast cancer who would not be treated with chemotherapy and for whom a homogeneous dose for the radiotherapy treatment plan could be achieved. The patient in this clinical scenario has numerous good clinical-pathologic risk features associated with her breast cancer—small tumor size, intermediate histology grade, hormone receptor responsive, low Oncotype score, age >60 years, and negative surgical margins—and she is committed to 5 years of an aromatase inhibitor for systemic therapy. Postlumpectomy treatment with 42.56 Gy in 16 fractions of HWBI, analogous to that of the Ontario Clinical Oncology Group (OCOG), is a reasonable and appropriate treatment option for this patient. In the OCOG study, none of the patients received a tumor bed boost, but the 10-year rate of in-breast recurrence was only 6.2% in the HWBI arm, suggesting that the potential benefit of a tumor-bed boost is small in this case. None of the randomized trials of HWBI studied the optimal boost delivery method, so no strong evidence exists for specific dose fractionation schemes in this setting. However, guidelines recommend that a sequentially delivered boost should be used if indicated and a dose of 10–16 Gy in 2 Gy fractions is reasonable.
Table 119.1 Phase III randomized controlled trials of hypofractionated versus conventional fractionated whole-breast irradiation after lumpectomy.
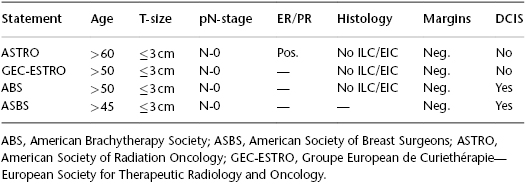
Accelerated partial breast irradiation (APBI) focuses a short hypofractionated course (typically, 5–10 treatments over 5–8 days) of postlumpectomy radiotherapy solely to the vicinity of the lumpectomy cavity. Analysis of in-breast recurrences following lumpectomy alone or with subsequent breast radiotherapy reveals that it more frequently occurs geographically within the vicinity of the original surgical cavity and much less frequently in other breast quadrants. This effectively defines a more focused target such that radiation aimed at this highest-risk region may account for the preponderance of radiation benefit in securing in-breast cancer control post lumpectomy. The findings from the European Organization for Research and Treatment of Cancer (EORTC) boost trial emphasize the importance of adequate radiation dose to the vicinity of the surgical cavity. A 40% relative reduction of in-breast recurrence was found when an additional boost dose to the lumpectomy cavity region to a cumulative amount of 66 Gy is given versus when just the whole-breast dose of 50 Gy is delivered. There are multiple methods for delivery APBI, including external-beam radiotherapy with 3DCRT or intensity-modulated radiation therapy, as well as brachytherapy with single-entry devices (e.g., MammoSite®, Contura®, or Savi®) or multicatheter implants. Numerous prospective phase II studies and retrospective case review data have demonstrated acceptable in-breast recurrence rates using APBI in select patients. However, evidence from randomized controlled trials comparing APBI to WBI (conventionally or hypofractionated) is not yet available. Three large multi-institutional randomized controlled trials have completed accrual, and two are still open for accrual. Until this level I evidence becomes available from clinical trial outcomes, numerous groups have issued statements and recommendations regarding which breast cancer patients can be safely treated post lumpectomy with APBI based on the existing data (Table 119.2). There is consistent agreement that breast cancer patients with positive nodes and tumor sizes >3 cm should not be treated with APBI. There is less agreement on whether younger women, triple-negative receptor status, and/or DCIS should be treated post lumpectomy with APBI prior to the outcome data from randomized clinical trials becoming available. McHaffie et al. (2011) studied 322 women who had received APBI with a multicatheter implant and who had been followed for a median of 60 months, and analyzed loco-regional recurrence rates (LRRs) by ASTRO consensus guideline groups. The 5-year LRR was as follows: suitable, 1.6% (95% CI: 0.0–4.8%); cautionary, 4.8% (95% CI: 0.7–8.9%); and unsuitable, 8.7% (95% CI: 3–14.4%). Among 104 patients with stage I or II invasive carcinoma, the actuarial LRR rate for patients considered cautionary by virtue of being age 50–59 alone is 0%, versus 12.7% in those deemed cautionary due to clinical or pathologic factors (P = 0.018). A suggestion of worse outcomes with adverse pathologic features is further supported by a recent update from the American Society of Breast Surgeons MammoSite® registry of single-entry balloon-based brachytherapy APBI for 1255 breast cancer cases. With a median follow-up of 60 months, ER-negative status was the only factor associated with in-breast recurrence for all patients with invasive carcinoma as well as for failures that occurred outside the treated APBI index region or “elsewhere” in the breast (P < 0.001). For patients with invasive malignancies, trends for increased rates of “elsewhere” recurrences in the treated breast were noted for increased tumor size (P = 0.067) and an extensive intraductal component (P = 0.087). Similarly, Pashtan et al. (2012) reported that of 98 women treated on a prospective trial of 3DCRT APBI at Massachusetts General Hospital with a 71-month median follow-up, 3 of 10 patients with triple-negative breast cancers experienced an in-breast recurrence, for a 5-year actuarial ipsilateral breast tumor recurrence rate of 33% (95% CI: 0–57%) compared to two of 88 patients without the triple-negative feature for a 5-year actuarial in breast recurrence rate of 2% (95% CI: 0–6%; P < .0001). Therefore, while the outcomes of randomized controlled trials are awaited, a conservative use of APBI in ASTRO consensus “suitable” patients is recommended with perhaps some accommodation of those aged 50–59 years, provided all other criteria are met. Therefore, this patient would also be a reasonable candidate for APBI as her sole radiotherapy modality post lumpectomy.
Table 119.2 ASTRO suitable, GEC ESTRO low risk, ABS and ASBS recommended criteria for treatment with APBI.
A survey in 2008–2009 of 1806 women from a mammography database from three hospitals (Wright-Patterson Medical Center, Wright-Patterson Air Force Base, Ohio; Clarian Health/Indiana University, Indianapolis, Indiana; and Wilford Hall Medical Center, Lackland Air Force Base, Texas) and 363 radiation oncologists from the ASTRO database evaluated patient and physician preferences regarding breast radiotherapy post lumpectomy. The patients’ order of preferences for radiotherapy was hypofractionated WBI (61.7%), followed by APBI (28.1%) and conventional fractionated WBI (10.1%). In contrast, the physician order of preferences was conventional WBI (81%), single entry brachytherapy APBI (55.5%), hypofractionated WBI (43.8%), 3DCRT APBI (35.9%), and multicatheter brachytherapy APBI (10.9%). With multiple choices of radiotherapy post lumpectomy, it is important for physicians to engage patients in selecting treatment approaches that maximize local control of the cancer and meet the patient’s individual needs.
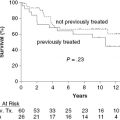
2. Can postlumpectomy breast radiotherapy be omitted for some breast cancer patients?