Case study 11.1
A 49-year-old male with no prior medical history was admitted to a department of internal medicine at a district hospital with a sore throat subsequent to several weeks of general fatigue and night sweats. Clinical biochemistry at admission revealed leukocytosis, anemia, and thrombocytopenia. He was referred to a university department, where a bone marrow (BM) aspirate and biopsy showed acute myeloid leukemia (AML) characterized by a large number of monocytoid cells, FAB-type M5. Cytogenetic examination showed a normal karyotype, and molecular testing revealed no AML-related fusion transcripts. However, overexpression of the Wilms tumor (WT1) gene could be demonstrated by quantitative PCR (qPCR) analysis. The patient received four courses of standard chemotherapy and achieved a complete morphological remission (CR) after the first course.
• Which techniques are available to detect residual leukemia in patients in CR?
In theory, any technique with sensitivity higher than that of classical immunohistochemistry will yield information regarding the depth of the achieved remission. The most commonly used techniques are qPCR and multicolor flow cytometry (MFC). While qPCR techniques have been extensively validated in multicenter efforts, less formalized testing has been performed with MFC. Minimal residual disease (MRD) detection using qPCR targets RNA or DNA sequences, which are derived from fusion genes, mutated genes, or genes overexpressed in AML cells. Fusion transcripts, arising from balanced translocations, have for some time been well known to be present in a minority of AML patients. Point mutations (e.g., in the NPM1 gene) are also an established qPCR target, whereas overexpression (e.g., of the WT1 gene) is considered a useful but less specific qPCR target. MFC–based MRD detection usually relies upon the aberrant immunophenotypic profiles of AML cells. Here, advantage is taken of the surface expression on the leukemic cells of leukocyte differentiation antigens present in either abnormal combinations or densities. This extent of aberrancy of the leukemic blasts is used to construct leukemia-associated immunophenotypes (LAIPs), namely, immunophenotypes that are rarely seen in healthy hematopoiesis but often characterize AML cells.
• Are MRD measurements of value in the evaluation of response to cytoreduction?
For all major MRD markers, prognosis has been shown to be affected by the MRD level after the first course of chemotherapy. There is therefore no doubt that MRD levels reflect a more accurate and biologically relevant way of judging the extent of disease disappearance. However, only a few trials studying AML have applied the consequence of these findings, adjusting treatment according to MRD measurements. Importantly, in one study of childhood AML, intensification upon demonstrating high levels of MRD at first-response evaluation resulted in superior overall survival, providing proof of principle for this approach. Of note, this decision making based on MRD was performed in patients in CR by standard techniques. More trials are underway to formally validate the concept of adjusting treatment intensity based on MRD status.
• Can MRD markers be identified for all AML patients?
The applicability of MRD markers varies considerably. WT1 is one of the most widely applicable MRD markers, because more specific targets are not seen in more than two-thirds of patient cohorts. Specific markers such as fusion transcripts are each rarely applicable in more than 5% of AML cases, whereas NPM1 mutation–based MRD measurement can be used in 35% of AML cases. The usefulness of the WT1 gene can be seen from reports stating that it is overexpressed in up to 70% of AML patients.
For MFC, MRD markers include the common aberrant immunophenotypes (e.g., CD7 expression on myeloblasts). Such markers can usually be found for more than 80% of AML cases; however, the search for LAIPs in each patient entails extensive testing.
• The patient is followed with standard hematological techniques and qPCR targeting WT1 every third month. The level of WT1 transcripts in peripheral blood (PB) has been normal for 3 years. For how long is continuous measurement of an MRD marker pertinent?
As can be seen from Figure 11.1, the majority of AML relapses occur within the first 2 years from diagnosis. That is not to say that relapse cannot occur later. However, on closer molecular characterization, late relapses will often turn out to be cases of secondary AML. The leukemic blasts of such patients are less likely to be recognized by measurements of the initial MRD marker. Many clinicians will thus opt to follow patients with MRD measurements for as long as clinical control is maintained, usually for 5 years. However, it might also be argued that cessation of MRD measurements (e.g., after 2 years) will be a welcome signal to the patient that the period of strict follow-up is over. Regardless, in terms of health economics, ending MRD follow-up after 2 to 3 years seems prudent.
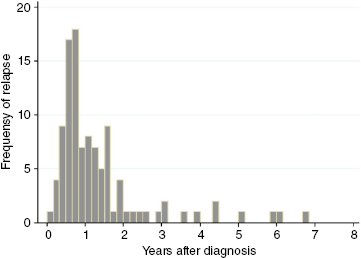 Figure 11.1 Time from end of chemotherapy to relapse in 84 Danish acute myeloid leukemia (AML) patients treated between 2000 and 2008.
Figure 11.1 Time from end of chemotherapy to relapse in 84 Danish acute myeloid leukemia (AML) patients treated between 2000 and 2008.



