FIGURE 16.1 Adjuvant treatment options for seminoma. XRT*, radiation therapy to para-aortic lymph nodes; BEP, bleomycin, etoposide, and ciplatin; EP, etoposide, and cipaltin.
DIAGNOSIS
The initial evaluation of a suspicious testicular mass should include measurement of serum tumor markers, testicular ultrasound, and a chest x-ray. Subsequently, a radical inguinal orchietomy should be performed. Post-operatively, if germ cell tumor is confirmed, abdominopelvic CT scan should be done and the tumor markers should be repeated if they were elevated prior to orchiectomy. Chest CT and brain imaging should be done if indicated.
Goals
■Every testicular mass requires a timely workup to exclude testicular carcinoma.
■Histologic determination of tumor type and stage has prognostic and therapeutic significance.
■Testicular cancer is highly curable, with 5-year survival >95%.
■Long-term sequelae of therapy should be considered and minimized when possible.
■Orchiectomy is essential for diagnosis and local tumor control. Radical inguinal orchiectomy is preferred to scrotal orchiectomy, trans-scrotal biopsy, or fine-needle aspiration due to the risk of scrotal violation that may compromise patients’ prognosis.
Laboratory
Serum α-Fetoprotein (AFP)
■A glycoprotein with a half-life of approximately 4 to 6 days
■Commonly produced by the fetal yolk sac, liver, and gastrointestinal tract
■Should not be elevated in serum of healthy men
■Not present in patients with pure seminoma. Elevated serum α-fetoprotein (AFP) levels indicate a nonseminomatous component to the patient’s testicular cancer
Serum α-Human Chorionic Gonadotropin
■Secreted by syncytiotrophoblasts; half-life of 0.5 to 1.5 days
■Most commonly elevated tumor marker in patients with testicular cancer
■Present in choriocarcinomas; may be modestly elevated in pure seminomas
■High levels may lead to gynecomastia
Serum Lactate Dehydrogenase
■Nonspecific tumor marker in testicular cancer
■Elevated in 80% of metastatic seminomas and 60% of advanced nonseminomatous tumors
■Reflects overall tumor burden, tumor growth rate, and cellular proliferation
Imaging
■Ultrasound: Ultrasound detects the presence of testicular parenchymal abnormality in both testes.
■Chest x-ray: Posterior–anterior and lateral film evaluation for pulmonary metastases.
■Computerized tomography (CT): CT scans of chest, abdomen, and pelvis determine extragonadal metastasis and are the most effective modality for staging the disease.
■Magnetic resonance imaging (MRI): MRI may provide additional information if ultrasound is indeterminate. MRI of the brain is necessary only when there are symptoms involving the central nervous system (e.g., headache, neurologic deficit, seizure).
■Positron emission tomography (PET) scan: PET scans are not indicated in primary staging, but may have limited utility for characterizing residual masses. The routine use of PET scans has not been shown to improve outcome.
Pathology
Patients with testicular masses should have surgical exploration, with complete removal of the testis and spermatic cord through the inguinal ring. Although empirical evidence supporting this is weak, trans-scrotal testicular biopsy is not recommended due to the risk of local and nodal dissemination of tumor.
■Immunohistochemical staining can be used to distinguish the different histologic subtypes of testicular carcinoma.
■Historically, CD30 and cytokeratin staining have been used to distinguish embryonal carcinoma (positive for both markers) from pure seminoma.
■Modern immunostains have made it possible to increase the accuracy of this distinction. NANOG and OCT3/4 are expressed in seminoma and embryonal carcinoma while SOX2 is only expressed in embryonal carcinoma.
■Seminoma expresses c-kit, which is expressed by neither embryonal carcinoma nor yolk sac tumor.
■SALL4, a novel stem cell marker, stains nearly all subtypes of germ cell tumors, making it a very useful marker in confirming metastatic disease. It is particularly useful for identifying yolk sac tumor, which is strongly positive for SALL4 staining but negative for OCT4.
■Germ cell tumors are frequently aneuploid and display an array of histopathology (Table 16.1).
■Several genes (either deleted or amplified) located on isochromosome 12p have been implicated in the malignant transformation of primordial germ cells. Among patients with familial testicular germ cell tumors compatible with X-linked inheritance, evidence suggests the presence of a susceptibility gene on chromosome Xq27.
STAGING
Staging is in accordance with the American Joint Committee on Cancer tumor/node/metastasis (TNM) criteria.
■T classification is based on pathologic finding after radical orchiectomy, hence the pT nomenclature. pT0 means there is no evidence of disease. pTis refers to intratubular germ cell neoplasia or carcinoma in situ. pT1 is a disease that is limited to the testis and epididymis without lymphovascular invasion, although it may invade the tunica albuginea but not the tunica vaginalis. pT2 tumor is similar to pT1 but with lymphovascular invasion or the involvement of the tunica vaginalis. In pT3 tumor, there is invasion of the spermatic cord with or without lymphovascular invasion. Involvement of the scrotum with or without lymphovascular invasion is designated as pT4. pTx is used when the primary tumor cannot be assessed.
■N classification may be pathologic (pN) or clinical. When there is no regional lymph node involvement the N0 designation is used. N1 refers to metastasis in a lymph node mass that is ≤2 cm in greatest dimension. N2 is a lymph node metastasis or multiple lymph nodes metastases with any one mass >2 cm but ≤5 cm. Lymph node metastasis >5 cm is termed N3. If lymph node metastasis is ascertained pathologically after surgery, then the pN nomenclature is used. pN0 means that there is no evidence of lymph node involvement, while pN1 is similar to N1 except that the involvement of ≤5 lymph nodes with none >2 cm in greatest dimension is also considered pN1. Likewise, pN2 is similar to N2 but also include the involvement of more than 5 lymph nodes, none more than 5 cm or evidence of extranodal extension. pN3 has similar definition as N3. When the regional lymph nodes cannot be assessed the Nx or pNx designation is used.
■M classification is based on the extent of distant metastasis. M0 means there is no distant metastasis, while M1, which is further divided to M1a and M1b, signifies distant metastasis. M1a refers to nonregional nodal or pulmonary metastasis, while M1b indicates the presence of nonregional nodal and nonpulmonary metastases.
■Very unique to testicular germ cell tumors is the use of serum tumor markers in the staging process. S0 refers to normal serum levels of tumor markers. S1 means that the lactate dehydrogenase (LDH) is <1.5 times the upper limit of normal, β-HCG is <5,000 milli-international units/mL, and AFP is <1,000 ng/mL. S2 is used when the LDH is between 1.5 and 10 times the upper limit of normal, or β-HCG is between 5,000 and 50,000 milli-international units/mL, or AFP 1000 to 10,000 ng/mL. S3 refers to LDH >10 times the upper limit of normal, or β-HCG >50,000 milli-international units/mL, or AFP >10,000 ng/mL. Sx refers to tumor markers not available or not done.
■The TNM classification is then used in the anatomic stage grouping as follows:
•Stage I: pT1-4, N0, M0, Sx/S0
•Stage IS: Any p T or Tx, N0, M0, S1-3
•Stage II: Any pT or Tx, N1-3, M0, Sx/S0-1
•Stage III: Any pT or Tx, any N, M1, Sx/S0-3
PROGNOSIS
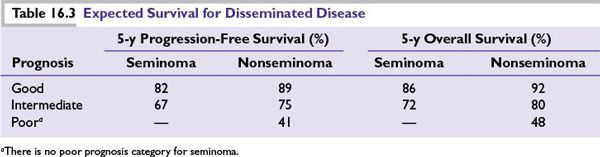
■The prognosis is based on the International Consensus Risk Classification system that utilized postorchiectomy levels of tumor markers and the site of metastasis to predict the progression-free survival (PFS) and overall survival (OS) of patients with advanced testicular germ cell tumors (Table 16.2).
■The 5-year PFS and 5-year OS for disseminated seminomatous and nonseminomatous germ cell tumors are given in Table 16.3.
TREATMENT MODALITIES
A radical inguinal orchiectomy is the preferred surgical approach for all patients with a testicular mass. This is both diagnostic and therapeutic. Adjuvant therapy, which may include chemotherapy, radiotherapy, or further surgery, is tailored to the disease stage and histology. Due to the unique radiosensitivity of seminomas, adjuvant radiation therapy is often employed. Patients should be counseled about sperm banking before the initiation of therapy. The need for aggressive therapy with early-stage disease is currently controversial.
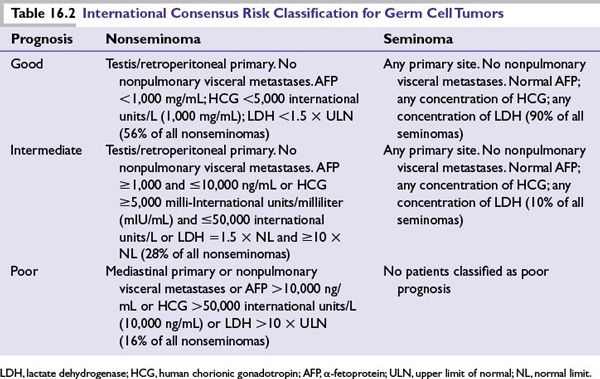
Seminomas
Adjuvant treatment options for seminoma are outlined in Figure 16.1.
Stage I
■Orchiectomy is curative for most patients with stage I seminoma. With a recurrence rate of up to 20%, active surveillance is an option after surgery for patients who can comply with follow-up recommendations.
■When disease does recur, usually in the retroperitoneal lymph nodes, nearly all patients can be cured with radiation or chemotherapy.
■For patients who cannot comply with active surveillance, low-dose radiation therapy to regional lymph nodes after orchiectomy results in cure over 90% of the time.
■Chemotherapy with carboplatin is an alternative adjuvant treatment option. A single cycle of carboplatin has proven to be equivalent to radiation in producing a high rate of relapse-free survival (RFS) and OS at 4 years. It is also associated with a lower risk of second germ cell tumor.
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree