Etiology
■Ultraviolet rays: Exposure to sun’s ultraviolet rays in the electromagnetic radiation spectrum is a major risk factor for melanoma development and is related to (Fig. 22.1)
–Intermittent intense exposure
–Exposure at a young age
–Exposure in individuals with fair skin, blue eyes, blonde or red hair, propensity for sunburns, and inability to tan (poor tanners)
■Age: High incidence in young and middle-aged adults as well as in older subjects.
■Sex: Slightly more common in male subjects than in females.
■Ethnicity: Higher incidence in Northern than in Eastern and Southern Europeans.
Familial Melanoma
■About 5% to 10% of melanomas are familial and up to 40% have hereditary basis.
■A tumor suppressor gene cyclin-dependent kinase inhibitor 2A (CDKN2A) is the most commonly mutated gene located on the short arm of chromosome 9.
■The protective effect of CDKN2A is mediated by encoded protein p16INK4A.
■Other candidate genes in this category include cyclin-dependent kinase 4 and CDKN2A/p14 alternate reading frame CDKN2A/ARF.
■A high-risk variant of the α-melanocyte-stimulating hormone receptor gene (MC1R) located on chromosome 16q24 and associated with red hair and freckles confer high risk of familial melanoma in families segregating the CDKN2A gene.
■Hereditary basis of melanoma should be suspected in the following circumstances:
•Individuals with three or more primary cutaneous melanomas
•Melanoma at young age and family history of melanoma (mean age between 30 and 40)
•Individuals with cutaneous melanoma and a family history of at least one invasive melanoma and two or more other diagnoses of melanoma and/or pancreatic cancer among first- or second-degree relatives on the same side of the family
•Melanoma associated in patients with dysplastic nevi and atypical nevi
■Precursor lesions of melanoma include
•Dysplastic nevi locus of which resides on short arm of chromosome 1
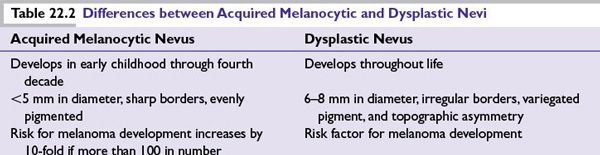
•Congenital nevi and acquired melanocytic nevi (Table 22.2)
Risk Factors for Melanoma
■Xeroderma pigmentosum
■Familial atypical mole melanoma syndrome (FAMMS)
■Advanced age and immune-suppressive states
■Sun exposure and sun-sensitive phenotype
■Melanoma in a first-degree relative and previous history of melanoma
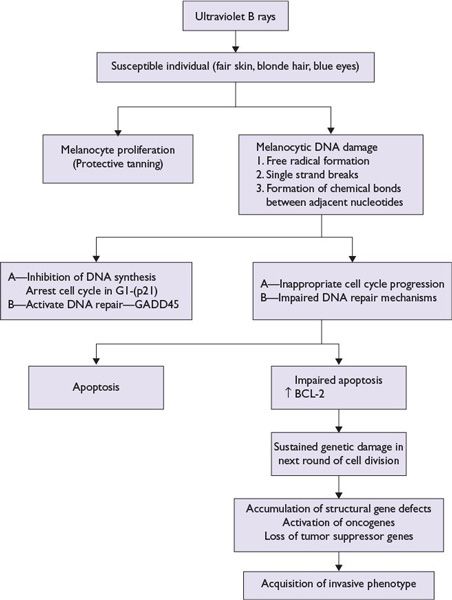
FIGURE 22.1 Model of ultraviolet B light-mediated pathogenesis of cutaneous melanoma.
Common Chromosomal Abnormalities in Melanoma
■Early chromosomal abnormalities:
•Loss of 10q
•Loss of 9p
■Late chromosomal abnormalities:
•Deletion of 6q
•Loss of terminal part of 1p
•Duplication of chromosome 7
•Deletion of 11q23
Clinical Features of Melanoma (abcde)
■Most cutaneous melanoma lesions are pigmented and display asymmetry, irregular borders, variegate colors with shades of brown, black or pink, white, red or blue, diameter of at least 6 mm; progressive change in size, nodularity, ulceration, or bleeding with or without pain and or itching and evolving in size, shape, or color.
■Less than 1% of cutaneous melanomas lack pigment. These are called amelanotic melanomas and may pose diagnostic challenges.
■Cutaneous melanoma can occur anywhere in the body. Cutaneous melanoma is more common in the lower extremities in women, the trunk in men, and head and neck in the elderly subjects.
Pathologic Diagnosis of Cutaneous Melanoma
Melanoma In Situ
Biopsy of a suspicious skin lesion for melanoma reveals characteristic tumor cell morphology in the basal epidermal layer verified by the tumor cells staining of melanoma-specific antigens such as S-100, premelanosomal protein HMB-45, nerve growth factor receptor, tyrosinase-related protein-1 (MEL-5). Melanoma tumor cells stain positive for vimentin and negative for cytokeratin stains.
Invasive Melanoma
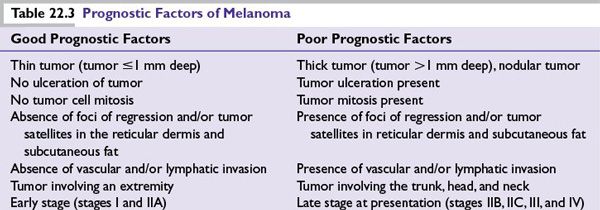
Tumor invasion into the dermis makes it an invasive melanoma quantified by two methods described by Clark et al. and Breslow see below. Additional histologic information provides important prognostic information and includes morphologic variants such as spindle cell morphology, ulceration, mitosis counted as number/mm2, lymphocytic infiltration, regression if any, vascular invasion, perineural invasion, and solar elastosis (Table 22.3).
Clinicohistologic Types of Melanoma: Microstaging
Clark Levels
Clark et al. subdivided melanoma invasion of the papillary dermis into a deep group in which tumor cells accumulate at the junction of the papillary and reticular dermis and a superficial group in which tumor cells did not invade deeper layers (Fig. 22.2).
Breslow Thickness
Breslow used an ocular micrometer to measure the vertical depth of penetration of tumor from the granular layer of the epidermis or from the base of the ulcerated melanoma to the deepest identifiable contiguous melanoma cell (Breslow thickness).
Principles of American Joint Committee on Cancer Melanoma Staging
Melanoma is staged based on information derived from three key categories (TNM):
■(T) Tumor characteristics on microscopic examination
■(N) Nodes—status of the regional lymph node metastasis
■(M) Distant metastasis—either present or absent
In American Joint Committee on Cancer (AJCC) staging, melanoma is divided into four stages:
■Stage I—thin melanoma (subdivided into IA and IB)
■Stage II—deeper melanoma without lymph node metastasis (subdivided into IIA, IIB, and IIC)
■Stage III—melanoma spread to regional lymph nodes (subdivided into IIIA, IIIB, and IIIC)
■Stage IV—distant metastasis (subdivided into M1a, M1b, and M1c)
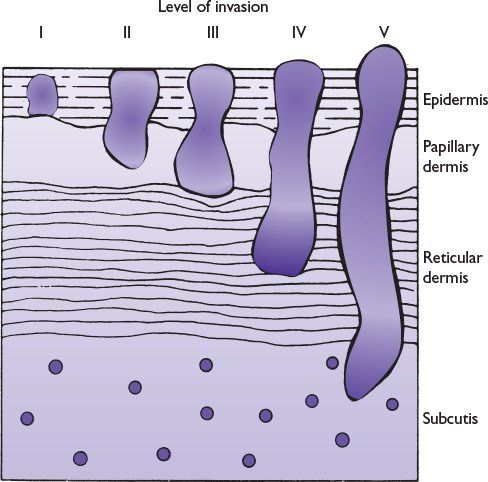
FIGURE 22.2 Schematic diagram of Clark levels of invasion.
Factors taken into consideration for subdividing each stage into subcategories A, B, or C include the following:
■Stages IA and IB: depth of invasion, ulceration, and mitosis
■Stages IIA, IIB, IIC: depth of invasion, presence or absence of ulceration
■Stages IIIA, IIIB, IIIC: Number of lymph node metastases, microscopic versus clinically palpable macroscopic lymph nodes, intralymphatic metastases (in transit metastasis) or satellites lesions (microscopic or macroscopic)
•A sentinel lymph node biopsy procedure is prerequisite to identify occult lymph node metastasis when involved lymph nodes are not palpable on clinical examination. Only exception is melanoma 1or less than 1 mm in depth without ulceration or mitosis.
•Immunohistochemical staining of melanoma-associated antigens is used to confirm morphologically identified cluster of few melanoma cells in the lymph node.
■Stage IV—M1a, M1b, M1c:
•M1a—Metastasis to the distant lymph node and subcutaneous tissues
•M1b—Lung metastasis
•M1c—Non–lung visceral metastasis that includes liver, bone, brain, and other organs
•Note: Serum enzyme lactate dehydrogenase if elevated upgrades M1a and M1b to M1c
Prediction of Patient Outcome Based on AJCC Melanoma Staging (Fig. 22.3)
■Low risk: Stages I and IIA (melanoma-specific mortality less than 25% at 20 years)
■Medium to high risk: Stages IIB, IIC, and III (melanoma-specific mortality between 55% and 75% at 20 years)
■Poor risk: Stage IV (melanoma-specific mortality more than 90% at 5 years)
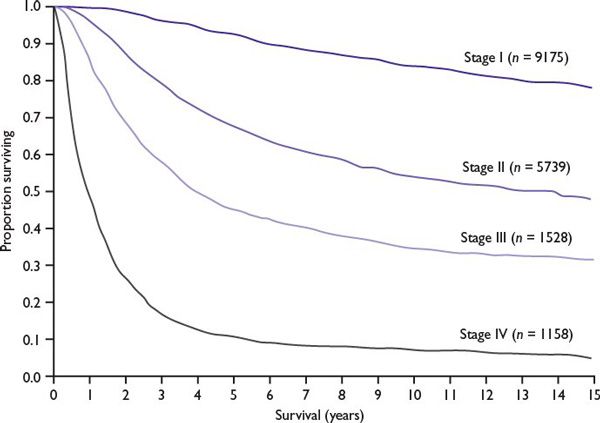
FIGURE 22.3 Relationship between the stage of melanoma and survival (20-year follow-up). (Kaplan-Meier survival curves adapted from Balch CM, Gershenwald JE, Soong S-J, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199-6206.)
Cutaneous Melanoma: Prevention and Early Diagnosis
■Patient education: increasing awareness of melanoma as a serious cancer, its risk factors, self-skin examination (SSE), sun avoidance, light clothing, and effective use of sunscreens.
■Close surveillance: Total body skin examination (TBSE) performed by a dermatologist provides close surveillance to identify suspicious skin lesions for biopsy and early diagnosis.
Selected patients at high risk of melanoma might benefit from the following two techniques:
■Digital photography is used for tracking suspicious skin lesions over time in patients with multiple nevi or dysplastic nevus syndrome.
■Dermoscopy (epiluminescence microscopy), performed by a trained operator, utilizes either a dermatoscope or 10× ocular scope (microscope ocular eyepiece held upside down) to visualize a variety of structures and patterns in pigmented skin lesions that are not discernible to the naked eye. The procedure has a potential to improve diagnostic sensitivity.
Melanoma Management: General Surgical Treatment Principles
An algorithm for melanoma management is presented in Figure 22.4.
Principle: Complete surgical excision of primary melanoma confirmed by comprehensive histologic examination of the entire excised specimen forms the basis of surgical treatment.
Risk of local recurrence: Relates to completeness of resection of the primary tumor, but is not significantly associated with the extent of surgical margin of excision (Table 22.4).
Assessment of the Regional Lymph Node Metastasis and Lymph Node Dissection
Principle: The risk of melanoma metastasis to the regional lymph nodes is directly proportional to the depth of invasion (>1 mm deep), tumor ulceration, and mitosis. For invasive melanoma less than or equal to 1 mm in depth, the risk is increased if ulceration or mitosis is present.
Regional lymph node metastasis: Reflects aggressive tumor biology and depth of dermal invasion that if left untreated pose serious risk of spread to adjacent lymph nodes or to systemic organs.
Historically, complete excision of primary cutaneous melanoma is followed with elective, therapeutic, or delayed lymph node dissection from the respective basin.
■Elective lymph node dissection: Although clinically not palpable, all the lymph nodes are dissected from the respective basin because of concerns of melanoma metastasis.
■Therapeutic lymph node dissection: Is performed if the regional lymph nodes are enlarged and clinically palpable (suspected lymph node metastasis).
■Delayed lymph node dissection: Is performed when initially nonpalpable regional lymph nodes become palpable over a follow-up period (delayed metastasis).
Lymph node dissection is recommended only if regional lymph node metastasis is present and is avoided if lack of lymph node metastasis could be predicted by a reliable test.
Sentinel Node Biopsy
■Sentinel lymph node biopsy as a tool to detect regional lymph node metastasis.
■Characteristics of a sentinel lymph node:
•First lymph node in the basin at greatest risk of metastasis.
•Easily accessible and identified by lymphoscintigraphy.
•Pathologic evaluation helps to detect occult melanoma lymph node metastasis.
■Surgical approach to obtain a sentinel lymph node: lymphoscintigraphy
•Preoperative lymphoscintigraphy uses vital blue dye injected around cutaneous melanoma that provides a road map of the lymph node basin. Intraoperative lymphoscintigraphy uses radio colloid injection around the primary tumor, and a handheld device detects the radioactivity from the involved lymph node. The combination of vital blue dye and technetium-labeled sulfur colloid helps the surgeon navigate the identity of sentinel lymph node in the respective nodal basin for metastasis in 94% of cases.
■Implications of sentinel lymph node biopsy:
•Complete lymph node dissection is recommended only if sentinel lymph node is positive.
•A negative sentinel lymph node saves the patient morbidity of this procedure.
•Sentinel lymph node biopsy–guided information about the extent of lymph node metastasis helps in prognostication of primary melanoma and reduces the risk of local recurrence. Its impact on overall survival is not clear.
•In one study, immediate lymph node dissection after positive microscopic metastasis in the sentinel lymph node conferred survival advantage in a subset analysis.
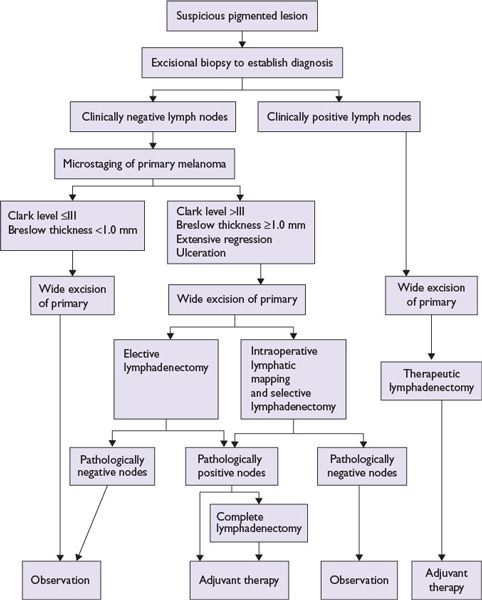
FIGURE 22.4 Algorithm for melanoma management.

Adjuvant Treatment of Melanoma in Patients at Risk of Recurrence after Surgery
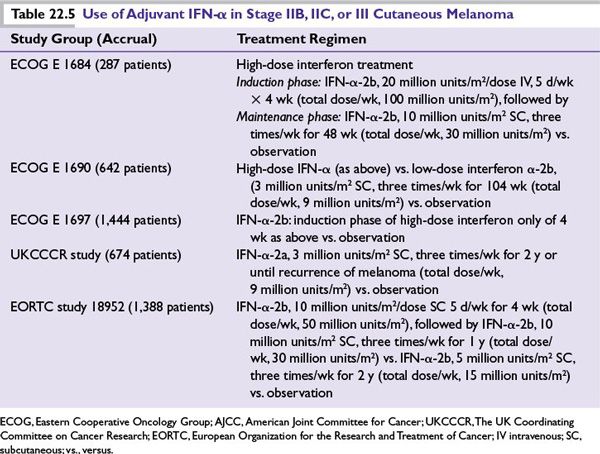
Interferon alpha (IFN-α) Treatment Principle: There is a high rate of relapse of cutaneous melanoma (35% to 75%) among patients with AJCC melanoma stages IIB, IIC, and III after primary surgical treatment. Based on antiproliferative and immunomodulatory effects, prolonged use of IFN-α (high dose, low dose, intermediate dose for variable periods of time) has been extensively studied in large prospective randomized clinical trials as an adjuvant to primary surgery to assess benefit of reducing recurrences and improving survival (Table 22.5).
■IFN-α treatment conferred consistent relapse and disease-free survival benefit.
■Impact upon overall survival has been variable and less consistent.
■High-dose IFN-α is superior when compared to low-dose.
•One month of high-dose intravenous IFN-α induction-only therapy is not sufficient and must be followed by the maintenance phase to confer any benefit.
•Pegylated form of IFN-α (slow release) given subcutaneously once weekly for up to 5 years conferred relapse-free survival advantage without overall survival benefit.
•The predominant toxicity of high-dose IFN-α severe flu-like symptoms, liver toxicity, and depression that adversely affect quality of life and may compromise the intended benefit of interferon therapy.
•The decision to use interferon in the adjuvant setting of cutaneous melanoma treatment should be based on the perceived relative merits of disease control, quality of life, and financial cost.
■Biochemotherapy as an adjuvant treatment of cutaneous melanoma after primary surgery did not confer survival advantage in one randomized phase 3 clinical trial.
■Ongoing clinical trials are evaluating the role of ipilimumab, an anti-CTLA antibody, and vemurafenib, an agent that targets BRAF mutation as adjuvant treatment of melanoma after primary surgery. Both of these agents have recently been approved for use in patients with metastatic melanoma.
Role of Radiation Therapy
■Pain relief of melanoma metastasis to the musculoskeletal region
■As an adjuvant treatment after the regional lymph node dissection:
•In melanoma of the head and neck region
•When lymph node metastases are bulky and/or involve four or more lymph nodes or exhibit extracapsular spread
•Local recurrence of melanoma in a previously dissected lymph node basin
■After surgical resection of desmoplastic melanoma with neurotropism
■Brain metastasis of melanoma
Radiation therapy of brain metastasis of melanoma includes
■Whole-brain radiation if multiple and large size brain metastases are present
■Stereotactic brain radiation is preferred if small-sized or isolated or fewer (two to three) brain metastases are present
ResuLts of the studies by Skibber et al. and others have suggested that external radiation to the brain after surgical resection of the solitary brain metastasis from malignant melanoma has survival benefits.
Isolated Limb Perfusion or Infusion as a Treatment of Melanoma
Principle: To deliver maximally tolerated chemotherapy doses in patients with locally advanced and metastatic melanoma to a regionally confined tumor area such as a limb while limiting systemic toxicity.
Isolated limb perfusion (ILP): Involves hyperthermia and oxygenation of the circulation that potentiate the tumoricidal effects of the chemotherapeutic agents that include melphalan (L-PAM), thiotepa, mechlorethamine with or without tumor necrosis factor (TNF-α), and IFNγ. This procedure provides palliative benefit to patients with in-transit metastasis of melanoma otherwise difficult to resect surgically or at high risk of recurrence after surgery (Table 22.6).
Isolated limb infusion: Is a simplified and minimally invasive procedure developed at the Sydney Melanoma Unit (SMU) intended to obtain the benefits of ILP without major disadvantages. It is a low-flow ILP procedure performed via percutaneous catheters without oxygenation.
Both the procedures provide local tumor control and pain relief with improvement of quality of life usually without impact on overall survival.

Management of Patients with Metastatic Melanoma
The management options for a patient with metastatic melanoma have expanded following the recent FDA approvals of two novel agents:
■Ipilimumab is a monoclonal antibody to CTLA-4 antigen on T lymphocytes that regulate T lymphocyte activation after an encounter with melanoma antigen.
■Vemurafenib is a targeted therapy that selectively blocks mutated BRAF in melanoma patients harboring BRAF mutation.
Both of these agents have shown overall survival benefits to patients with metastatic melanoma and have unique toxicity profiles (see below).
■High-dose interleukin-2 (IL-2) (see below).
■Surgical resection of an accessible isolated metastatic lesion has a curative potential in about 25% of patients, while those who are not candidates for complete surgical resection of a metastatic lesion will require systemic treatment.
■Chemotherapy as a single agent, in combination, or with biologic agents (biochemotherapy) in patients with metastatic melanoma.
■Experimental therapies such as adaptive cell therapy and newer targeted therapies still remain promising options for selected patients with metastatic melanoma.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree