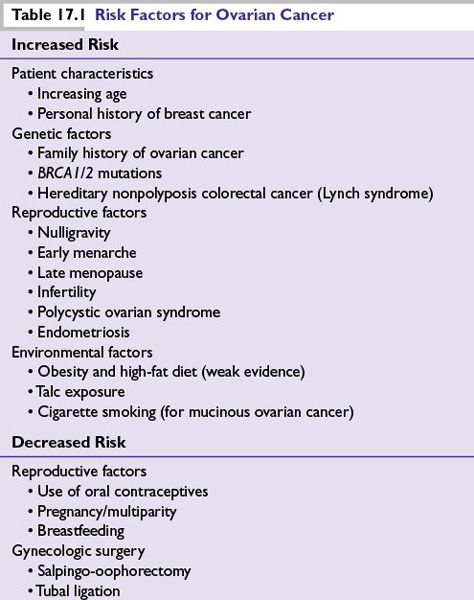
FIGURE 17.1 A flow chart for ovarian cancer FIGO staging. Patients are staged at diagnosis based on the extent of spread of the ovarian cancer. Correct staging is critical as it impacts treatment decisions.
RISK FACTORS
■Table 17.1 lists risk factors for ovarian cancer.
PREVENTION
■The use of oral contraceptives is protective against EOC for the general population. Increasing duration of use is associated with larger reductions in EOC risk.
■Risk reduction salpingo-oopherectomy (RRSO) has been shown to reduce the lifetime risk of ovarian/tubal/peritoneal cancer in high-risk women to less than 5%. RRSO is recommended for high-risk women, those with familial ovarian cancer syndromes and/or BRCA1/2mut. Surgery is recommended after completion of childbearing and, where feasible, approximately 10 years earlier than the age of diagnosis of the youngest affected family member.
■RRSO has been shown to decrease the risk of breast cancer up to 50% in BRCA1/2mut patients.
■RRSO is not recommended for women at average risk.
SCREENING
■The 2012 Reaffirmation Recommendation Statement of the U.S. Preventive Services Task Force reiterated its recommendation against screening for EOC in women who are asymptomatic and without known genetic mutations that increase its risk.
•Mounting evidence suggests that annual screening with transvaginal ultrasonography (TVU) and serum cancer antigen 125 (CA-125) does not reduce mortality. High false-positive rates leading to intervention are associated with subsequent harm, such as unnecessary surgical intervention.
•Women with a family history of breast/ovarian cancer should be offered genetic counseling. Refer to the NCI website for details: http://www.cancer.gov/cancertopics/factsheet/Risk/BRCA
■Familial ovarian cancer syndrome patients and known BRCA1/2mut carriers who have not undergone RRSO may be offered screening consisting of a pelvic examination, TVU, and a CA-125 blood test every 6 months beginning between the ages of 30 to 35 years, or 5 to 10 years earlier than the earliest age of first EOC diagnosis in the family. There are no data demonstrating survival benefit of screening high-risk patients.
■Women with high-risk families in whom deleterious mutations are not found (BRCA1/2, Lynch syndrome–associated genes) are treated similarly to those in whom genetic risk is identified. RRSO is recommended; absent RRSO, screening as for high-risk women is reasonable.
SERUM BIOMARKERS
■CA-125 is a high-molecular-weight glycoprotein and marker of epithelial tissue turnover produced by ovarian, endocervical, endometrial, peritoneal, pleural, colonic, and breast epithelia.
•CA-125 is increased in ~50% of early-stage and >90% of advanced stage EOC. It is elevated most commonly in serous histology.
•Specificity of CA-125 for ovarian cancer is poor. It can be increased in many benign conditions, such as endometriosis, first trimester pregnancy, pelvic inflammatory disease, uterine fibroids, benign breast disease, cirrhosis, and in response to pleural or peritoneal effusions of any cause, and other epithelial malignancies.
•CA-125 is FDA approved for use as a biomarker for monitoring EOC response to treatment and recurrence. It is neither approved nor recommended for screening.
•The reliability of following CA-125 concentrations during molecularly targeted therapy is unknown.
■Human epididymis protein 4 (HE4) is a glycoprotein also expressed in some EOC. It is increased in >50% of tumors that do not also express CA-125.
•HE4 testing is FDA approved as a biomarker for monitoring EOC recurrence and response to treatment. It is neither approved nor recommended for screening.
DIAGNOSIS AND EVALUATION
■EOC is not a silent disease. Symptoms are present, though nonspecific.
■Several studies suggest usefulness of a symptom index tool to identify women who may have EOC: new (within 1 year) and persistent (more than 12 times/month) pelvic/abdominal pain, increased abdominal size/bloating, difficulty eating/feeling full, and urinary urgency/frequency should trigger evaluation by a gynecologic oncologist.
■Stromal tumors can produce virilization, precocious puberty, amenorrhea, and/or postmenopausal bleeding, depending on patient age, and type and amount of ectopic hormone produced.
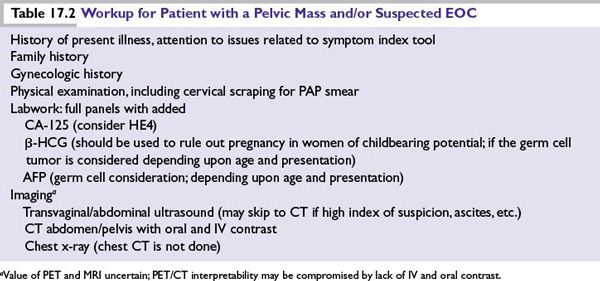
■The preoperative workup of a patient with a suspected ovarian malignancy is summarized in Table 17.2.
■Early referral to a gynecologic oncologist is recommended. The extent and quality of surgical debulking have important prognostic value and are an integral part of the upfront management of an EOC patient.
TREATMENT
Surgery
■Proper EOC diagnosis and staging require laparotomy with en bloc TAH/BSO tumor removal, abdominal fluid sampling, tumor debulking, and pathologic assessment of the abdomen, including diaphragms, paracolic gutters, and serosal surfaces. Unilateral salpingo-oophorectomy can be considered in women with stage I grade 1/2 tumors who wish to preserve fertility. Completion of salpingo-oopherectomy is recommended upon completion of child-bearing.
■The primary goal of EOC surgery is complete debulking. Data indicate better outcome for women undergoing surgical debulking by a gynecologic oncologist.
■Optimal debulking, defined as <1 cm maximal diameter residual disease, has positive prognostic value; no visible residual disease yields an even better prognosis.
■Stage I disease with favorable prognostic features (grade 1/2, stage IA/B, non–clear cell histology) can be treated by surgery alone.
■Complete primary debulking surgery (PDS) is still considered the gold standard in the United States. Neoadjuvant chemotherapy (NACT) followed by surgery or with interval debulking surgery yields similar overall survival (OS) to PDS.
■Second-look laparoscopy/laparotomy is no longer supported in the United States.
Initial Chemotherapy
■Stage IC disease with favorable prognostic features can be treated by surgery followed by limited course platinum-based chemotherapy (three cycles; GOG-157); six cycles are recommended for poor prognosis feature stage IC.
■The recommended standard of care adjuvant therapy for optimally debulked advanced stage III patients is a combination of IV paclitaxel and intraperitoneal (IP) cisplatin/paclitaxel chemotherapy. It is unclear if the benefit was due to the dose density, site of administration, or both factors.
■The standard of care NACT or adjuvant therapy for patients with suboptimally debulked stage III/any stage IV disease and patients who cannot tolerate IP chemotherapy is six cycles of IV carboplatin and IV paclitaxel every 21 days.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree