Diagnosis
■A diagnosis of hypercalcemia is primarily made from serum levels of calcium (Table 36.2). Testing of ionized calcium may be indicated in some settings.
■The calcium concentration [Ca] usually changes by 0.8 mg/dL for every 1.0 g/dL change in plasma albumin concentration.
■Formula for corrected serum calcium concentration:
•(mg/dL) = serum Ca (measured) + 0.8 × [4- serum albumin concentration (g/dL)]
■In hypercalcemia of malignancy, serum intact parathormone (iPTH) level is low or undetectable.
General Principles of Treatment
■Unfortunately, hypercalcemia most often occurs in advanced stages of disease and in patients who have progressed through available standard chemotherapy. In patients with solid tumor primary cancers, survival is often <6 months after hypercalcemia is diagnosed.
■Any symptomatic patient with hypercalcemia, regardless of absolute serum calcium level, should be treated for correction of the hypercalcemia.
■Symptomatic patients with severely elevated calcium levels often require profound fluid volume replacement, which makes outpatient therapy impractical and unsafe.
■Mild asymptomatic hypercalcemia with serum calcium concentration in the range of 11 to 12 mg/dL should be treated, when there is associated hypercalciuria, because of the risk of nephrolithiasis and nephrocalcinosis.
Practical Management
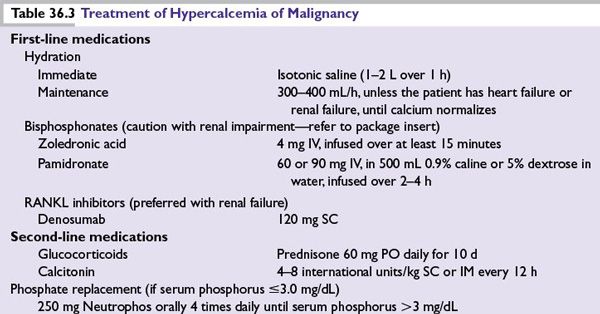
■Immediate administration of isotonic saline (1 to 2 L over 1 hour followed by 300 to 400 mL per hour, unless the patient has heart failure or renal failure) to increase renal blood flow and calcium excretion.
■Once rehydration is complete and urinary output is optimized, the need for bisphosphonate administration should be assumed.
■IV zoledronic acid (4 mg IV, infused over at least 15 minutes) is commonly used in malignancy-induced hypercalcemia. Usually a single dose is adequate, when used to treat hypercalcemia.
■Bisphosphonate administration is well tolerated by patients except for occasional IV site irritation and fever during infusion. Its onset of action is within 24 to 48 hours of administration; the maximal effect may not be achieved until 72 hours after treatment.
■Dose should only be repeated after at least 7 days.
■The ASCO guidelines for bisphosphonate use in myeloma recommend that for zoledronic acid,
•Creatinine clearance >60 mL per minute; no dosing changes are required.
•Creatinine clearance >30 mL per minute and <60 mL per minute; dose should be reduced (follow package insert).
•Creatinine clearance <30 mL per minute contraindicated.
■Denosumab inhibits osteoclast development, activation, and survival by preventing the receptor activator of nuclear factor-kappa B ligand and has been used to treat bisphosphonate refractory hypercalcemia. In meta-analysis of over 5,000 patients there was an increased incidence of hypocalcemia in the denosumab group; grade 3 or 4 laboratory abnormalities for hypocalcemia were 88 (3.1%) for the denosumab group and 38 (1.3%) for the zoledronic acid group. In addition, denosumab is safe to be used in patients with renal failure and could be effective in bisphosphonate refractory patients.
■Denosumab should be the preferred agent in patients with creatinine clearance <30 mL per minute.
■Depending on clinical urgency, dental evaluation must be obtained before bisphosphonates are initiated to prevent osteonecrosis of the jaw.
■Osteonecrosis of the jaw is a potentially devastating complication of bisphosphonate and RANKL inhibitor use, and is associated with poor dentition. Among bisphosphonates use, jaw osteonecrosis rates are higher among myeloma patients. Patients with multiple myeloma had a rate 4.5 times that of patients with breast cancer in one study.
■Corticosteroids can be considered in select patients and is often effective. These tumors include lymphoma, leukemia, myeloma (prednisone, 40 to –100 mg per day), and breast cancers (prednisone, 15 to 30 mg per day) during hormonal therapy.
■Calcitonin has a rapid onset of action (within 4 hours) and is often useful in severe and symptomatic hypercalcemia until the more slowly acting agents become effective (e.g., zoledronic acid, pamidronate, and gallium nitrate).
■Salmon calcitonin is initially given at 4 units per kg (body weight) SC or IM every 12 hours. If response is not satisfactory after 1 to 2 days, the dosage may be increased to 8 units per kg SC or IM every 12 hours. If response is still not adequate after a 1- to 2-day trial at the higher dose, the dosing interval should be decreased to 8 units per kg SC or IM every 6 hours. Although many patients initially will respond to calcitonin, tachyphylaxis often develops rapidly, which renders patients refractory to its hypocalcemic effect.
■Hemodialysis should be considered, in addition to the other treatments listed for hypercalcemia, in patients who have serum calcium level in the range of 18 to 20 mg/dL and/or in those who have neurologic symptoms but are hemodynamically stable.
■Galium nitrate and mithramycin have been found to be useful in the treatment of malignancy associated hypercalcemia.
■Treatment of hypercalcemia of malignancy is summarized in Table 36.3.

TUMOR LYSIS SYNDROME
Tumor lysis syndrome (TLS) occurs when cellular disruption results in life-threatening lactic acidosis, with concomitant hyperuricemia, hyperkalemia, hyperphosphatemia, and hypocalcemia.
Etiology
■Tumor lysis can occur before the start of treatment (primary TLS) or more commonly after the administration of highly effective therapy (secondary TLS). The risk of developing TLS depends on multiple factors such as disease burden and preexisting nephropathy. In one study, for AML patients, tumor lysis was the major cause of death in 2% of cases.
Clinical Setting, Signs, and Symptoms
■TLS typically occurs in patients with acute leukemias (AML and ALL) with high white cell count, though it can also occur in patients with lymphomas (particularly Burkitt lymphoma) and solid tumors that are sensitive to therapy.
■TLS can be classified as laboratory TLS and clinical TLS. Laboratory TLS is defined as two or more abnormal values of uric acid, potassium, phosphorus, or calcium at presentation or a 25% change from baseline.
■Clinical TLS is defined as laboratory TLS accompanied with seizures, cardiac arrythmias, renal dysfunction, or sudden death.
■These criteria must be met 3 days before and up to 7 days after the initiation of therapy.
■Clinical index of suspicion should be high, as onset of TLS could be insidious. Cardiac arrhythmias may result from the severe hyperkalemia or hypocalcemia that accompanies the TLS. Hypocalcemia can result in tetany, whereas hyperphosphatemia and hyperuricemia can result in acute renal failure (ARF).
Management
Main Principles
■Identification of high-risk patients with initiation of preventive therapy.
■Early recognition of metabolic and renal complications with prompt supportive care, including hemodialysis.
Prevention and Treatment (Table 36.4)
■Preventive measures include the identification of individuals at risk; 24 to 48 hours of vigorous pretreatment volume expansion (3,000 mL/m2/day), use of pretherapeutic allopurinol (300 to 600 mg, PO q day), and vigilant metabolic monitoring (every 3- to 4-hour laboratory tests) after institution of therapy. These actions are the hallmarks of TLS prevention and management. Elevated levels of lactate dehydrogenase (LDH), uric acid, or creatinine at presentation identify a particularly high-risk patient.
■Rasburicase is a recombinant urate oxidase that catalyzes enzymatic oxidation of poorly soluble uric acid into an inactive and more soluble metabolite (allantoin). It can be given as a single dose between 3 mg and 7.5 mg. The manufacturer suggested dose is 0.2 mg/kg as an IV infusion over 30 minutes. Usually a single dose is adequate, though as per the manufacturer up to five doses can be administered.
■Rasburicase enzymatically degrades uric acid in blood samples left at room temperature. Blood should be collected in prechilled tubes containing heparin, transported in an ice bath, and assayed within 4 hours.
■Rasburicase should not be administered to patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency.
HYPERPHOSPHATEMIA
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree