LUNG CANCER SCREENING
■Randomized trials of screening with chest radiography with or without sputum cytology have shown no reduction in lung cancer–related mortality.
■Low-dose computed tomography (CT) screening may benefit individuals at an increased risk for lung cancer. The potential harms of screening and the generalizability of results are unclear.
•The National Lung Screening Trial (NLST), a randomized trial, compared annual screening by low-dose chest CT scanning with chest x-ray for 3 years in high risk individuals (age between 55 and 74 years with at least 30 pack-year cigarette smoking, and former smokers who had quit within the previous 15 years: n = 53,454). There were 247 deaths from lung cancer per 100,000 person-years in the low-dose CT group and 309 deaths per 100,000 person-years in the radiography group, representing a relative reduction in mortality from lung cancer with low-dose CT screening of 20.0% (95% CI 6.8 to 26.7; P = 0.004). The rate of death from any cause was reduced in the low-dose CT group, compared with the radiography group by 6.7% (95% CI 1.2 to 13.6; P = 0.02).
CLINICAL PRESENTATION
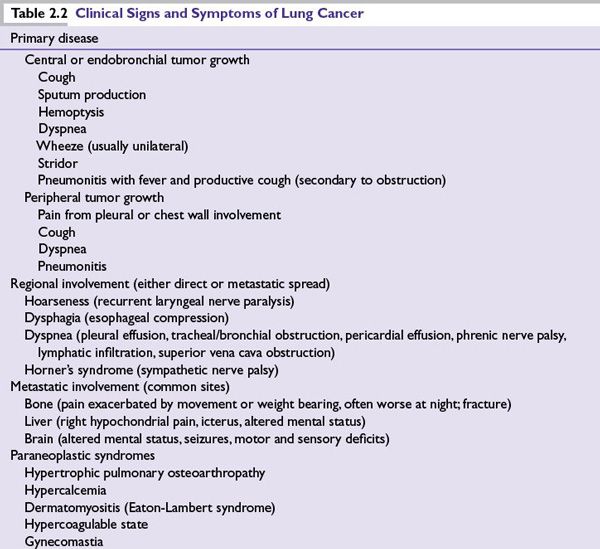
■A minority of patients present with an asymptomatic lesion discovered incidentally on chest radiograph. No set of signs or symptoms are pathognomonic of lung cancer, so diagnosis is usually delayed.
■Clinical signs and symptoms of lung cancer are outlined in Table 2.2.
CLINICAL EVALUATION
Single Pulmonary Nodule
■Definition: Solitary mass, often found incidentally, surrounded by lung tissue, well circumscribed, measures <3 cm without mediastinal or hilar adenopathy.
■Benign inflammatory vascular abnormalities or infectious lesions can mimic more sinister lesions. Review of previous chest imaging is a crucial first step. A stable lesion over a 2-year period suggests a benign condition.
■CT of the chest is required to assess for other nodules, adenopathy, or chest wall invasion.
■18F-fluorodeoxyglucose-positron emission tomography (“FDG-PET”) is used to evaluate single pulmonary nodules (SPNs). False-positive PET scans may occur in conditions such as tuberculosis or histoplasmosis. False-negative results have been reported for small lesions (<1 cm) and neoplasms with low metabolic activity, such as in some cases of BAC. Mean sensitivity of FDG-PET is 96%; mean specificity is 75%. The negative and positive predictive value of PET for pulmonary nodules is approximately 90%.
■A growing SPN needs a pathologic diagnosis. Tissue can be obtained by fine needle aspiration (FNA), transbronchial biopsy, or surgical resection. Flexible fiber optic bronchoscopy is appropriate for central lesions and can lead to a diagnosis in 97% of cases via biopsies, bronchial washings, and brushings.
■Observation may be reasonable in a low-risk individual (<40 years old and has never smoked) with a negative FDG-PET and a stable lesion measuring <2 cm. Reimaging with regular CT scans and follow-up clinic appointments are recommended.
Suspected Lung Cancer
■Full history and physical examination are recommended, followed by complete blood count and chemistry tests, chest x-ray, and CT of the chest and abdomen (including adrenal glands).
■Sputum analysis may be helpful in cases of central lesions.
■Bone scans and plain films of affected areas are warranted where bone pain exists. Routine imaging of the brain in asymptomatic patients is controversial.
■Peripheral lesions may require percutaneous transthoracic FNA, which can be performed under CT or fluoroscopic guidance.
■Mediastinoscopy, a more invasive method, may be needed to obtain a histologic diagnosis in difficult-to-reach primary tumors. Mediastinoscopy can reveal unsuspected tumors in mediastinal lymph nodes—a negative implication for survival. Evaluation of the mediastinum is recommended before surgery in suspected mediastinal disease and intraoperatively prior to any planned resections.
■An accurate pathologic diagnosis and staging of disease is essential in the management of lung cancer. Stage of disease determines whether surgical resection is warranted. Clinical staging often underestimates the true extent of the disease. The combination of PET evaluation and mediastinoscopy is routinely used to complete staging.
■Preresection forced expiratory volume per second (FEV1) should be ≥2 L for pneumonectomy, 1 L for lobectomy, or 0.6 L for segmentectomy.
■Preresection forced vital capacity should be ≥1.7 L.
■In patients who undergo surgical resection, surgical/pathologic staging should be used to predict recurrence and to evaluate the need for adjuvant therapy.
STAGING
■The tumor-node-metastasis (TNM) staging system bases patient prognoses on tumor size, lymph node involvement, and metastasis. Median overall survival for patients with pathologic stages IA, IB, IIA, IIB, IIIA, IIIB, and IV are 119, 81, 49, 31, 22, 13, and 17 months, respectively.
■The seventh edition of the TNM Classification of Malignant Tumours (UICC) was adopted by the American Joint Committee on Cancer (AJCC) in 2010. A summary of the TNM classification, stage grouping, and anatomical drawing can be found at http://www.cancerstaging.org/staging/posters/lung12x15.pdf. In stages I and II, disease is limited to one lung and does not involve the mediastinum or more distant sites. Involvement in stage III is heterogeneous and ranges from a tumor of size ≤ 2 cm with metastasis in ipsilateral mediastinal and/or subcarinal lymph node (T1a, N2-stage IIIA) to a tumor of any size with local invasion or a separate nodule in a different ipsilateral lobe with metastasis in contralateral mediastinal or hilar nodes (T4, N3-stage IIIB). Stage IV includes tumor involvement in a contralateral lobe and presence of malignant pleural (or pericardial) effusions or distant metastases.
TREATMENT
Stages I and II
■Stage I and stage II NSCLCs are considered early-stage disease. These two stages combined account for 25% to 30% of all lung cancers.
■Five-year survival rates are 58% to 73% for stage I and 36% to 46% for stage II.
■Surgical resection is the recommended treatment for patients with stage I and stage II NSCLCs. In patients who are medically fit for surgical resection, lobectomy or greater resection is recommended rather than sublobar resections (wedge or segmentectomy).
■Video-assisted thorascopic surgery (VATS) is an acceptable alternative to open thoracotomy.
■Intraoperative systematic mediastinal lymph node sampling or dissection is recommended for accurate pathologic staging.
■Even with complete resection, approximately half of these patients eventually experience relapse after resection, with a two- to three-fold higher proportion of distant metastases over local recurrences.
•In selected patients who undergo complete surgical resection, several large trials have demonstrated a statistically significant survival benefit from cisplatin-based adjuvant chemotherapy (IALT, ANITA, JBR 10).
•The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis which used individual patient data (n = 4,584) from five trials with a median follow-up of 5.2 years found that adjuvant cisplatin-based chemotherapy was associated with a an decrease in absolute risk of death of 5.4 % at 5 years compared with no chemotherapy (hazard ratio [HR] 0.89; 95% CI 0.82 to 0.96).
■Among completely resected early-stage NSCLC, adjuvant chemotherapy is not recommended for stage IA, is standard for stage II, and may be useful in a subset of patients with stage IB.
•In the LACE meta-analysis, the overall survival benefit varied considerably by stage of disease, with potential harm seen in stage IA (HR 1.40; 95% CI 0.95 to 2.06), a trend toward benefit in stage IB (HR 0.93; 95% CI 0.78 to 1.10), and clear benefit in stage II (HR 0.83; 95% CI 0.73 to 0.95) patients.
■Since there is no reliable way to identify which stage IB patients may derive benefit from adjuvant chemotherapy, current guidelines recommend chemotherapy in stage IB high-risk patients, defined by large size (more than 4 cm), poor differentiation, vascular invasion, visceral involvement, and suboptimal resection.
■Current evidence suggests that postoperative radiotherapy is associated with decreased survival for patients with stage I (N0) and stage II (N1) NSCLCs. However, most meta-analyses included several older studies that used radiotherapy methods that are inferior to current methods.
■If surgery is contraindicated in early-stage NSCLC, radiotherapy can be an effective means of local control. In clinical studies, accelerated radiotherapy (54 Gy in 12 days) was associated with better 4-year survival than conventional radiotherapy (60 Gy in 6 weeks). Stereotactic body radiation therapy (SBRT), which delivers a high dose to a target volume and spares surrounding normal tissues, may be an option for patients with primary tumors of size <5 cm and in whom surgery is contraindicated.
Stage IIIA
■Stage IIIA (N2) NSCLC is a therapeutically challenging and controversial subset of lung cancer, with a 5-year survival rate of only 24%.
■Randomized trials strongly suggest a combined modality approach in stage IIIA disease. Conflicting data, however, have led to difficulties in proposing specific management guidelines. This, in part, is secondary to the heterogeneous nature of stage IIIA disease.
■Clinically N0 or N1 patients are often taken for upfront surgical resection with cure achievable in 25% to 50% of these patients. However, should incidentally discovered N2 disease be found at surgery, complete tumor resection and mediastinal lymphadenectomy are recommended. With the high rate of recurrence in this patient population adjuvant chemotherapy to address micrometastatic disease is recommended.
•The International Adjuvant Lung Cancer Trial of 1,867 patients with stages IB to IIIA (39% stage IIIA) randomized patients to three to four cycles of postoperative cisplatin-based chemotherapy versus surgery alone, with adjuvant 60 Gy radiotherapy given to both arms of stage IIIA patients (the use of radiotherapy was left to the investigator’s choice). After a median 56-month follow-up, the overall survival rate was significantly higher in the chemotherapy group (HR 0.86), with a 5-year survival rate of 44.5% in the chemotherapy group versus 40.4% in the control arm, with the strongest benefit in patients with stage III disease.
•The ANITA study randomized 840 completely resected patients with stages I to IIIA (35% stage IIIA) to four postoperative cycles of cisplatin and navelbine versus observation (radiotherapy as per preference of participating center). After a median follow-up of >70 months, long-term 5-year survival of stage IIIA patients in the chemotherapy arm was significantly greater at 42% versus 26% in the observation arm (P = 0.013).
■Postoperative radiation therapy (PORT), while reducing local recurrence, does not improve survival, may be detrimental, and is not recommended as standard of care. Advocates of radiotherapy have emphasized that there are several differences between the treatment administered in several trials included in this meta-analysis and current practices in the United States.
•The PORT meta-analysis (Meta-Analysis Trialist Group) of 2,128 patients treated in nine randomized trials with a median follow up of 3.9 years found a significant increase in risk of death with PORT (overall risk ratio 1:21; P = 0.001).
■Evidence has yet to be established substantiating the benefit of adding adjuvant radiotherapy to adjuvant chemotherapy in fully resected stage IIIA patients.
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree