CLINICAL FEATURES
Bone pain, particularly in the back or chest, and less often in the extremities, is present in nearly 60% of patients with MM. Patients may present with pathologic fractures and can also have loss of height because of vertebral collapse. Other common clinical features include fatigue (32%), weight loss (24%), normocytic normochromic anemia (73%), and hypercalcemia (28%). MM can also result in a low anion gap due to severe hypercalcemia and/or the cationic immunoglobin molecule. Renal insufficiency is seen in almost half the patients with MM at diagnosis and is commonly caused by hypercalcemia and related dehydration, and light chain cast nephropathy. Other etiologies may include renal amyloidosis, light chain deposition disease, cryoglobulinemia, or drug-induced kidney injury. In some patients, amyloidosis can cause a nephrotic syndrome (<5%). Acquired Fanconi syndrome with glycosuria, phosphaturia, and aminoaciduria can also occur with MM. MM patients are at an increased risk for infection due to impaired lymphocyte function, suppression of normal plasma cell function, and hypogammaglobulinemia. Patients can also present with radiculopathy or spinal cord compression that can result from compression of nerve roots by paravertebral plasmacytoma or by fractured vertebral body. Peripheral neuropathy is a rare manifestation and, when present, is almost always secondary to amyloidosis.
DIAGNOSIS AND WORKUP
Diagnosis of MM requires evidence of a clonal plasma cell disorder with the presence of end-organ damage (hypercalcemia, renal insufficiency, anemia, or bone lesions) attributable to the plasma cell disorder. The criteria for diagnosis of monoclonal gammopathies proposed by the International Myeloma Working Group (IMWG) are shown in Table 27.1. When MM is suspected, the diagnostic workup should include a thorough history and physical examination with specific attention to complaints of bone pain, constitutional symptoms, neurologic symptoms, and infections. In addition, for diagnosis and staging, these labs should be performed: complete blood count with differential; serum electrolytes, blood urea nitrogen, serum creatinine, calcium, phosphate, magnesium, uric acid, albumin, β2-microglobulin, and lactate dehydrogenase; serum protein electrophoresis (SPEP) and immunofixation (IFE); serum FLC assay; 24-hour urine protein electrophoresis (UPEP) and IFE; quantitative immunoglobulins; radiographic skeletal survey; and bone marrow aspirate and biopsy.
SPEP is useful in detecting and quantifying the presence of an intact monoclonal protein (M-protein) that is visualized as an M-spike in the gamma region. Serum IFE confirms the presence of the monoclonal immunoglobulin and, more importantly, determines its type (Fig. 27.1). SPEP and/or serum IFE is sometimes inadequate as approximately 15% of patients have only light chains (light chain myeloma), which may rapidly be cleared from the plasma to the urine. Hence, serum FLCs, UPEP, and/or urine IFE should be performed in all patients and are very useful in such patients.
SPEP detects an M-spike in 82% of patients with MM. Addition of serum IFE increases the sensitivity to 93%. The sensitivity increases to 97% or more if either the serum FLC assay or 24 hour UPEP/urine IFE is performed in addition. Patients who lack detectable M-protein by any of these tests, but have end-organ damage and clonal plasma cells in the bone marrow, are considered to have nonsecretory myeloma. The circulating M-protein on IFE is IgG in 52% of cases, IgA in 21%, light chain only (kappa or lambda) in 16%, IgD in 2%, and biclonal in 2%. IgM myeloma is exceedingly rare and is seen in <1% of cases. Kappa is the predominant light chain isotype compared with lambda (ratio 2:1), except in IgD myeloma, where lambda isotype is more common.
Bone marrow studies should include conventional karyotyping and fluorescent in situ hybridization (FISH) designed to detect t(11;14), t(4;14), t(14;16), t(6;14), t(14;20), hyperdiploidy, and deletion 17p for risk stratification. Gene expression profiling, when available, may also be considered for additional prognostic information.
Radiologic changes seen on a skeletal survey include punched-out lytic lesions, severe osteopenia or osteoporosis, and pathologic fractures. A nuclear medicine bone scan is not useful in MM because lytic lesions are not visualized on bone scans. Routine fluoro-deoxyglucose positron emission tomography/computed tomography (PET-CT) and magnetic resonance imaging (MRI) scans are not needed for every patient, but are indicated when symptomatic areas show no abnormality on a radiographic skeletal survey or when there is uncertainty about the true extent of bone disease on radiographs alone. Another indication where these scans should be utilized is when solitary plasmacytoma is suspected, to reliably rule out bony or extramedullary disease. Any patient with significant back pain should also undergo MRI of the spine to evaluate cord compression.
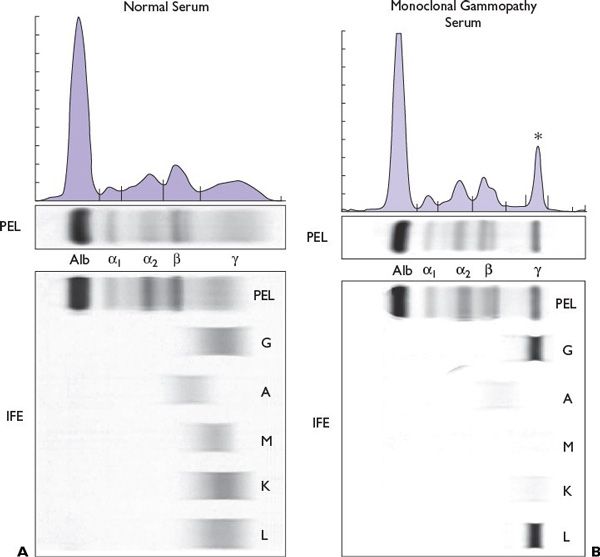
FIGURE 27.1 Electrophoretic pattern of (A) normal human serum and (B) immunoglobulin G (IgG lambda) multiple myeloma. Asterisk indicates M spike in the gamma region.
STAGING
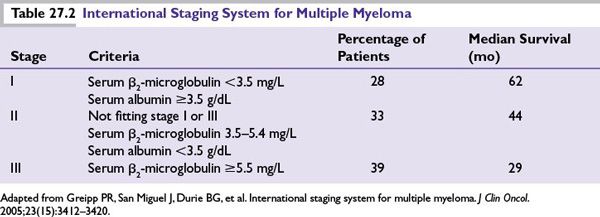
Two main staging systems exist for MM that primarily reflect tumor burden: the International staging system (ISS) that is based on laboratory values and the Durie-Salmon staging system, predominantly a clinical system. Both of these provide prognostic information but are not helpful in making therapeutic choices. Of these, the ISS has become the preferred staging system because of its simplicity and lack of subjectivity (Table 27.2).
PROGNOSIS
Prognosis in myeloma depends on host factors (age, performance status, and comorbidities), stage, disease aggressiveness, and response to therapy. Other laboratory parameters such as hemoglobin concentration, creatinine, calcium, lactate dehydrogenase, immunoglobulin subtype, plasmablastic morphology, circulating plasma cells, and C-reactive protein have also been shown to be independent risk factors for survival in myeloma. A high plasma-cell–labeling index also strongly predicts poor prognosis, but this test is not commonly available. A risk stratification model based on independent molecular cytogenetic markers to assess disease aggressiveness has been found useful for both prognosis and therapeutic decision-making. Newly diagnosed patients can be stratified using these markers as having standard-, intermediate-, and high-risk disease based on the Mayo stratification for myeloma and risk-adapted therapy (mSMART) classification (Table 27.3). Median survival varies from 8 to 10 years for standard-risk patients versus 2 to 3 years for high-risk myeloma.
TREATMENT
General
Monoclonal Gammopathy of Undetermined Significance
Risk-stratification models have been proposed for progression of monoclonal gammopathy of undetermined significance (MGUS) and assist in detecting patients with higher risk of progression to myeloma. Patients with risk factors consisting of a serum M-protein >1.5 g/dL, IgA or IgM MGUS, and an abnormal serum FLC ratio have a risk of progression at 20 years of 58%; compared with 37% when two risk factors are present; 21% when one risk factor is present; and only 5% when none of the risk factors are present. Patients with MGUS should be monitored indefinitely without treatment because 20% to 25% of them will eventually progress to myeloma at a rate of approximately 1% per year. Patients should be followed with SPEP every 6 months, and if stable can be followed every year for high or intermediate-risk patients and every 2 to 3 years for low-risk patients (no risk factors present) or when myeloma symptoms arise. Treatment is not indicated unless it is part of a clinical trial.

Smoldering (Asymptomatic) Multiple Myeloma
These patients should also be observed closely without therapy, but they have a higher risk of progression to myeloma than MGUS (10% per year vs. 1% per year). These patients should have SPEP, UPEP, complete blood count, and calcium and creatinine measurement 2 to 3 months after the initial diagnosis. If the results are stable, the studies should be repeated every 4 to 6 months during the first year and, if stable, evaluation can be lengthened to every 6 to 12 months. Patients with an abnormal FLC (involved to uninvolved) ratio of >100 have 72% risk of progression to MM in the first 2 years after diagnosis and should be monitored more closely. Currently, treatment is indicated only when there is evidence of progression to symptomatic disease, or as a part of clinical trial, although there is increasing thought that treating high risk patients early (before they develop symptomatic disease) may lead to better outcomes.
Solitary Plasmacytoma
These patients are treated with radiation therapy (solitary bone) and/or surgical removal (extraosseous plasmacytomas) of the affected area, followed by close monitoring of M-protein every 6 months because of the risk of developing MM.
Multiple Myeloma
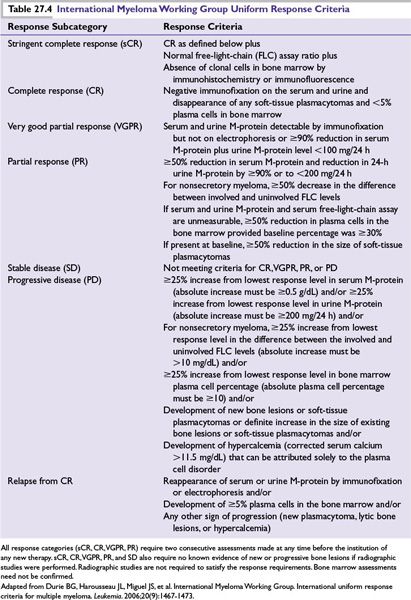
To date, there is no clear curative therapy available for most MM patients and the “cure vs. control” debate is ongoing. In the past decade, the availability of novel highly active drugs such as thalidomide, bortezomib, and lenalidomide has significantly improved the outcome of patients with MM. More recently newer drugs like carfilzomib and pomalidomide have become available for management of relapsed disease. One approach is upfront aggressive multidrug treatment to achieve complete response (CR) versus a sequential disease control approach emphasizing quality of life and prolonged survival. Patients with high-risk disease have better long-term OS if they achieve a CR, justifying an aggressive strategy upfront. For standard-risk patients, achievement of CR does not affect OS and the goal of therapy is to improve quality of life, delay disease progression, and prolong survival. The treatment choice for symptomatic myeloma patients largely depends on eligibility for transplantation and risk stratification (Fig. 27.2). Eligible patients should always be considered for enrollment in clinical trials that evaluate novel treatment strategies. The proposed criteria by the IMWG for evaluating disease response and progression in myeloma patients are outlined in Table 27.4.
Initial Therapies
Induction Treatment for Patients Eligible for Transplantation
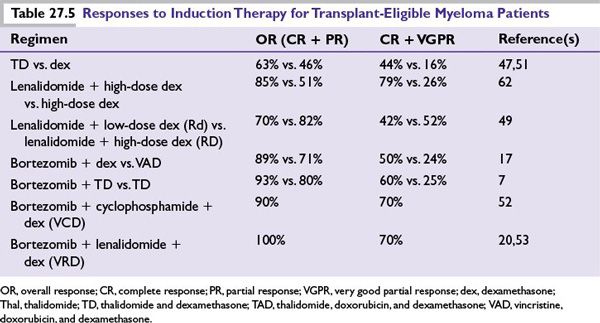
Infusional therapy with vincristine, doxorubicin, and dexamethasone (VAD) was commonly used for many years as an induction regimen prior to ASCT. However, VAD is no longer used as initial therapy since several novel drug combinations using thalidomide, bortezomib, or lenalidomide have been shown to be superior to VAD. A summary of these regimens is shown in Table 27.5.
Thalidomide-Dexamethasone This combination has been shown in randomized trials to have higher response rates and improved time to progression as compared to dexamethasone alone. Still, due to inferior activity and toxicity profile as compared to lenalidomide-based regimens, thalidomide-dexamethasone (TD) is not used as front-line therapy, except where lenalidomide may not be available. Thalidomide also has utility in patients with renal failure as no dose adjustments are needed and it can be safely combined with bortezomib. The combination of thalidomide, cyclophosphamide, and dexamethasone has been studied in phase 3 trials, and is an effective initial therapy. Patients are at high risk of developing venous thrombosis and should be on DVT prophylaxis with aspirin, low-molecular-weight heparin, or warfarin.
Lenalidomide-Based Regimens Lenalidomide is a safer and more effective analog of thalidomide, which in combination with dexamethasone has been superior to dexamethasone alone in randomized trials. Combination of lenalidomide with low-dose dexamethasone (40 mg once weekly) (Rd) is significantly less toxic and provides better OS as compared to combination with high-dose dexamethasone (RD). Long-term results suggest an excellent toxicity profile with prolonged therapy with this regimen. All patients should be given antithrombosis prophylaxis with aspirin. Low-molecular-weight heparin and warfarin should be used in patients at high risk of thrombosis. Lenalidomide has been combined with bortezomib as well as older drugs resulting in very active combinations both for initial therapy and for relapsed disease.
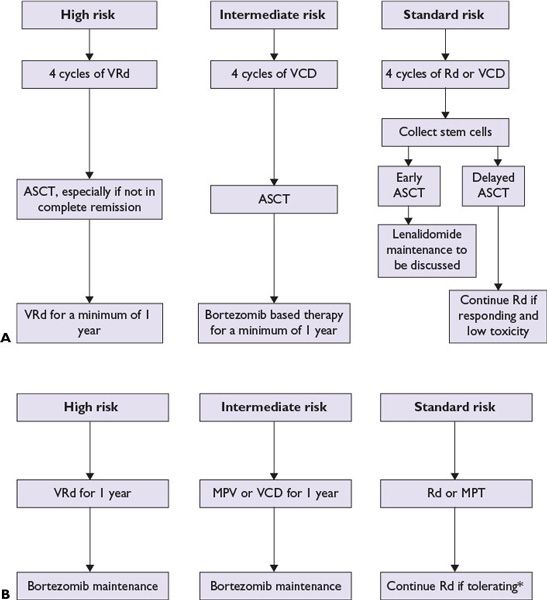
FIGURE 27.2 A suggested treatment algorithm for newly diagnosed multiple myeloma patients. Transplant eligible (A) and transplant ineligible (B). All patients should receive supportive care and must be considered for bisphosphonate treatment and clinical trials. (*Dexamethasone is usually discontinued after 12 months. ASCT, autologous stem cell transplantation; VRd, bortezomib, lenalidomide, dexamethasone; VCD, bortezomib, cyclophosphamide, dexamethasone; Rd, lenalidomide, dexamethasone; MPT, melphalan, prednisone, and thalidomide; MPV, melphalan, prednisone, and bortezomib; CR, complete response; VGPR, very good partial response.) (Adapted from Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095-1110; Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8:479-491.)
Bortezomib-Based Regimens Randomized trials have shown that bortezomib in combination with dexamethasone (VD) is superior to VAD as pretransplant induction therapy. Three drug combinations containing bortezomib such as combination with thalidomide and dexamethasone (VTD), cyclophosphamide and dexamethasone (VCD), and lenalidomide and dexamethasone (VRD) are highly active in newly diagnosed MM. VTD has been shown to be superior to TD and VD (Table 27.5). VCD is less expensive and better tolerated than VRD with similar activity in newly diagnosed patients. It also appears to overcome the poor prognosis associated with t(4;14) and hence is an excellent choice as front-line therapy for intermediate-risk patients. These three drug combinations have not been directly compared to Rd. Peripheral neuropathy is a significant adverse effect, which may occur early in the disease course with upfront bortezomib-containing regimens. Administering Bortezomib subcutaneously (as compared to intravenously) and on a once-weekly schedule significantly reduces the risk of neuropathy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree