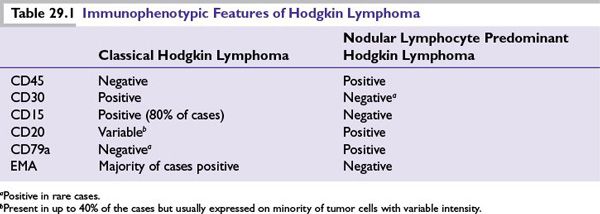
FIGURE 29.1 (A) Diagnostic Reed-Sternberg (RS) cell, seen in classic types of Hodgkin lymphomas (mixed cellularity, nodular sclerosis, lymphocyte depletion). (B) Variants of RS cells seen in nodular lymphocyte-predominant Hodgkin lymphomas: popcorn cells or L and H cells (lymphocytic or histiocytic predominance). RS cells of the classic type generally are not seen in a nodular lymphocyte-predominant Hodgkin lymphoma.
Pathologic Classification
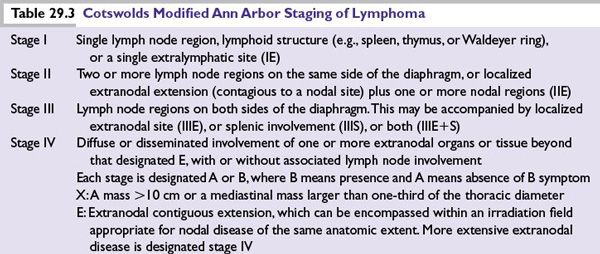
The World Health Organization (WHO) classification divides HL into two main types (Table 29.1):
■CHL
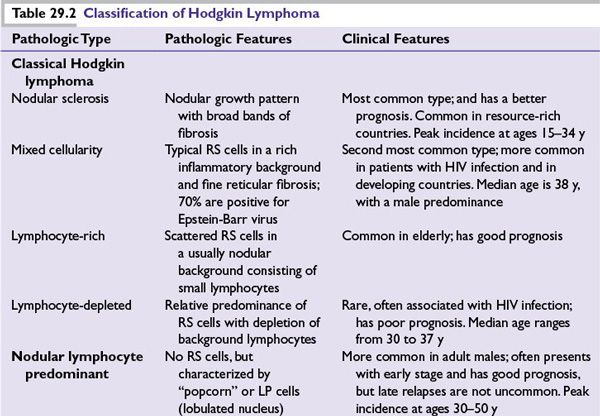
•CHL is characterized by the presence of RS cells in an inflammatory background and is divided into four histologic subtypes.
–Nodular sclerosis HL
–Mixed cellularity HL
–Lymphocyte-rich HL
–Lymphocyte-depleted HL
■NLPHL
•NLPHL lacks RS cells but is characterized by LP cells, which are sometimes referred to as popcorn cells.
Table 29.2 summarizes the clinical and pathologic features of the disease subtypes.
CLINICAL FEATURES
■Lymphadenopathy: Most commonly above the diaphragm (cervical, axillary, or mediastinal). Enlarged nodes are not tender with a characteristic firm rubbery consistency. Lymph node pain may occasionally be precipitated by alcohol intake.
■Chronic pruritus.
■Most common extranodal sites of involvement are lung, bone marrow, liver, and bones.
■B symptoms.
–Unexplained weight loss (>10% body weight over 6 months before diagnosis)
–Fever of >38°C, intermittent with 1- to 2-week cycles
–Drenching night sweats
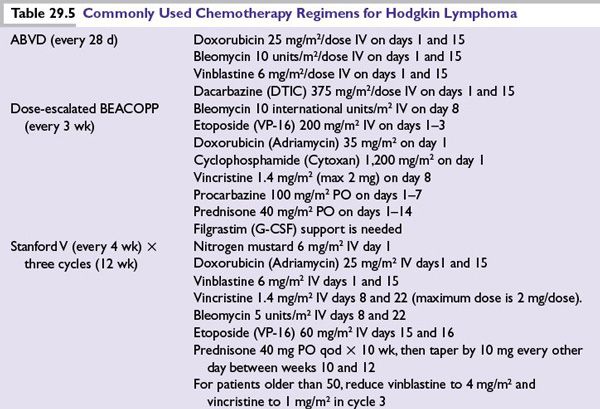
Staging
The modified Ann Arbor staging of lymphoma is used to clinically stage HL (Table 29.3).
Diagnostic Evaluation
Excisional biopsy of an enlarged lymph node is strongly recommended for initial diagnosis. A core biopsy may be appropriate if adequate tissue can be obtained to avoid major surgery. A fine-needle aspiration is not recommended for initial diagnosis.
Laboratory Tests
■Complete blood count (CBC), differential, and platelets.
■Erythrocyte sedimentation rate (ESR): Adverse prognostic biomarker, if elevated.
■Lactate dehydrogenase (LDH) and albumin.
■Liver function tests: If abnormal, may be associated with liver involvement.
■Alkaline phosphatase: May be nonspecifically high or associated with bone involvement.
■BUN, creatinine, electrolytes, and uric acid.
■Pregnancy test: Women of childbearing age.
■HIV testing in patients with risk factors for HIV.
Radiologic Studies
■Chest radiograph.
■Computerized tomography (CT) scan of the chest, abdomen, and pelvis are required for staging. CT-scan of the neck may sometimes be needed.
■Positron emission tomography (PET) CT-scan.
Unilateral Bone Marrow Biopsy and Aspiration
Required in clinical stage IB, IIB, III, or IV.
Evaluation/Procedures for Specific Treatments and Counseling
■MUGA scan or echocardiography to evaluate left ventricular ejection fraction before anthracycline treatment.
■Pulmonary function tests (including DLCO) are recommended prior to bleomycin-containing treatment.
■Fertility counseling (to discuss sperm, ovarian tissue, and/or oocyte cryopreservation).
■Smoking cessation counseling.
■Vaccination (pneumococcal, hemophilus influenza, and meningococcal) prior to splenic irradiation is recommended.
MANAGEMENT
■HL is sensitive to radiation and many chemotherapeutic agents. All patients, regardless of stage, should be treated with a curative intent. Cure rates are high (>80%), thus limiting long-term toxicities is a major consideration of treatment.
■Early-stage disease may be treated with combined-modality chemotherapy and radiation treatment (RT), or chemotherapy alone.
■Advanced-stage disease is usually treated with chemotherapy alone.
■In advanced-stage disease radiation consolidation can be considered for PET-positive areas following a full course of chemotherapy, but should be omitted in patients with PET-negative residual masses. Based on pre-PET era studies, routine radiation consolidation in patients with bulky (≥10 cm or one-third the diameter of the chest on CXR) disease is widely practiced in North American centers; however, radiation consolidation may not be necessary in PET-negative bulky masses.
Principles of Chemotherapy
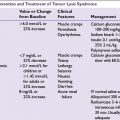
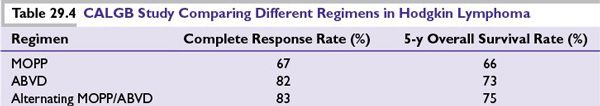
■The standard regimen for HL in North America is ABVD since it superseded MOPP regimen in the large randomized trial of the Cancer and Leukemia Group B (CALGB) in 1992 (Table 29.4). ABVD was associated with less myelosuppression and reduced risk of secondary leukemias and infertility compared to MOPP regimen. Growth factors are not required with ABVD. Treatment delay and/or dose reduction due to leukopenia is not recommended.
■The German Hodgkin Lymphoma Study Group (GHSG) developed the dose-escalated BEACOPP regimen and showed it to be superior to COPP-ABVD and standard-dose BEACOPP in advanced HL. However the significant associated toxicities of dose-escalated BEACOPP (3% rate of treatment-related death, 2% to 3% rate of secondary leukemias, and nearly universal infertility) has precluded its widespread use in North America. Dose-escalated BEACOPP is not recommended for elderly HL patients (≥60 years).
■Stanford V is a dose-intense 12-week regimen. Involved field radiation to macroscopic splenic disease and all lymph nodes measuring ≥ 5 cm in size is an integral part of Stanford V. The cumulative doses of doxorubicin and bleomycin in Stanford V are less than those in ABVD, with potentially less risk for cardiac and pulmonary toxicity. In the three randomized prospective trials (from Italy, United Kingdom, and United States) compared to ABVD, Stanford V had inferior complete remission rates and was associated with more hematologic and neurologic toxicity.
Chemotherapy regimens are described in Table 29.5.
Principles of Radiotherapy
■Radiation therapy for HL targets sites with either clinical disease (involved field or involved nodal) or involved plus adjacent areas (extended field). Extended fields are either “mantle field” for the cervical, axillary, and mediastinal regions or “inverted Y field” for spleen, para-aortic, and pelvic regions. When inverted Y field radiation is given together with mantle field radiation, the combination is called total nodal radiation.
■Dose of RT depends on the extent of the disease. In combined-modality therapy, RT is initiated ideally within 3 weeks of finishing chemotherapy.
Treatment Response Evaluation
All patients (early and late stages) should receive interim restaging (after two and/or four cycles of chemotherapy) to evaluate the response to treatment. Restaging should be repeated 3 months after the end of treatment if complete remission is not achieved in the interim assessment.
TREATMENT OF EARLY DISEASE (STAGES I AND II)
Early CHL should be treated with intent to cure. Poor risk factors have been identified in this subset of patients.
GHSG Unfavorable Prognostic Features for Early-Stage Disease (I and II)
Any of the following four features:
■Extranodal disease
■Bulky disease: A mass >10 cm in diameter or high mediastinal mass ratio (> one-third of maximum intrathoracic diameter)
■ESR >50 with no B symptoms, or >30 with B symptoms
■More than 2 nodal areas
Early-stage patients with bulky disease and stage IIB patients are best treated like advanced-stage (stage III/IV) disease. The remaining early-stage patients can be managed as following:
■Favorable early disease (by GHSG criteria): These patients are treated with ABVD × two cycles followed by 20 Gy of involved field radiation. The cure rate of these patients is >90%.
■Unfavorable early disease (by GHSG criteria): These patients are treated with ABVD × four cycles followed by 30 Gy of involved field radiation.
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree