■Initial upper gastrointestinal endoscopy and double-contrast barium swallow identify suggestive lesions and have diagnostic accuracy of 95% and 75%, respectively, but add little to preoperative staging otherwise.
■Endoscopic ultrasonography assesses the depth of tumor invasion (T staging) and nodal involvement (N staging) with accuracies up to 90% and 75%, respectively.
■Computerized tomographic scanning is useful for assessing local extension, lymph node involvement, and presence of metastasis, although understaging occurs in most cases.
■Although whole-body 2-[18F]fluoro-2-deoxyglucose (FDG)–positron emission tomography (PET) may be useful in detecting metastasis as part of preoperative staging in some gastric cancer patients, the sensitivity to detect early-stage gastric cancer is only about 20% and overall appears less reliable than in esophageal cancer.
PROGNOSIS
Pathologic staging remains the most important determinant of prognosis (Table 5.1). Other prognostic variables that have been proposed to be associated with an unfavorable outcome include the following:
■Older age
■Male gender
■Weight loss greater than 10%
■Location of tumor
■Tumor histology: diffuse versus intestinal (5-year survival after resection, 16% vs. 26%, respectively); high-grade or undifferentiated tumors
■Four or more lymph nodes involved
■Aneuploid tumors
■Elevations in epidermal growth factor or P-glycoprotein level
■Overexpression of ERCC1 and p53; loss of p21 and p27
MANAGEMENT OF GASTRIC CANCER
Standard of Care
Although surgical resection remains the cornerstone of gastric cancer treatment, the optimal extent of nodal resection remains controversial. The high rate of recurrence and poor survival of patients following surgery provides a rationale for the use of adjuvant or perioperative treatment. Adjuvant radiotherapy alone does not improve survival following resection. In addition to complete surgical resection, either postoperative adjuvant chemoradiotherapy (chemoRT) or perioperative polychemotherapy appear to confer survival advantages. The results of the Intergroup 0116 study show that the combination of 5-fluorouracil (5-FU)-based chemoRT significantly prolongs disease-free and overall survival when compared to no adjuvant treatment. Similarly, the use of polychemotherapy pre- and postoperatively can increase disease-free and overall survival compared to observation.
In advanced gastric cancer, chemotherapy enhances quality of life and prolongs survival when compared with the best supportive care. There are numerous therapeutic options in this setting without a clear standard of care. Of the commonly used regimens, triple combination chemotherapy with either docetaxel, cisplatin, and 5-FU (DCF) or epirubicin, oxaliplatin, and capecitabine (EOX) probably has the strongest claims to this role for the majority of fit patients. However, there is a pressing need for assessing new agents, both cytotoxic and molecularly targeted, in the advanced and adjuvant settings.
Resectable Disease
Surgery
Complete surgical resection of the tumor and adjacent lymph nodes remains the only chance for cure. Unfortunately, only 20% of US patients with gastric cancer have disease at presentation amenable to such therapy. Resection of gastric cancer is indicated in patients with stage I to III disease. Tumor size and location dictate the type of surgical procedure to be used. An exploration to exclude carcinomatosis just prior to the definitive resection is justified in this disease. Current surgical issues include subtotal versus total gastrectomy (TG), extent of lymph node dissection, and palliative surgery.
Subtotal versus TG Subtotal gastrectomy (SG) may be performed for proximal cardia or distal lesions, provided that the fundus or cardioesophageal junction is not involved (Fig. 5.1). TG is more appropriate if tumor involvement is diffuse and arises in the body of the stomach, with extension to within 6 cm of the cardia. TG is associated with increased postoperative complications, mortality, and quality-of-life decrement, necessitating thorough consideration of complete gastric resection (Fig. 5.2).
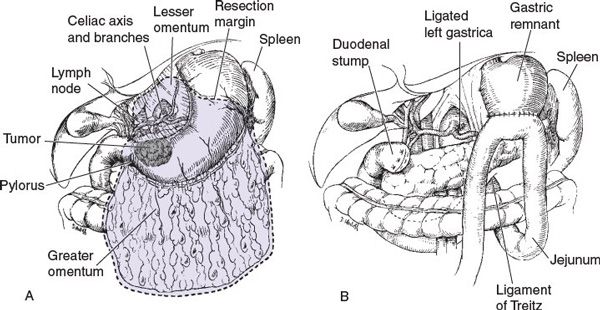
FIGURE 5.1 Subtotal gastrectomy.
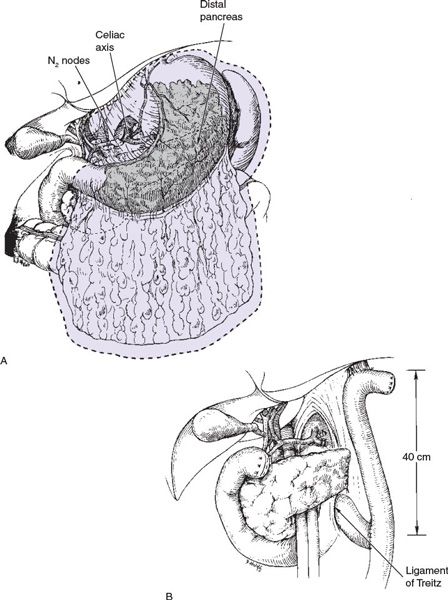
Extent of Lymph Node Dissection Regional lymph node dissection is important for accurate staging and may have therapeutic benefit as well. The extent of lymphadenectomy is categorized by the regional nodal groups removed (Table 5.2). At least 16 lymph nodes must be reported for accurate AJCC staging. D2 lymphadenectomy is reported to improve survival in patients with T1, T2, T3, and some serosa-involved (currently T4a) lesions as compared to D1. However, factors such as operative time, hospitalization length, transfusion requirements, and morbidity are all increased. The routine inclusion of splenectomy in D2 resections is no longer advocated given higher postoperative complications. The greatest benefit of more extensive lymph node dissection may occur in early gastric cancer lesions with small tumors and superficial mucosal involvement as up to 20% of such lesions have occult lymph node involvement.
Radiation Therapy
■For patients with locally advanced or metastatic disease, moderate doses of external-beam radiation can be used to palliate symptoms of pain, obstruction, and bleeding, but do not routinely improve survival.
■Local or regional recurrence in the gastric or tumor bed, the anastomosis, or regional lymph nodes occurs in 40% to 65% of patients after gastric resection with curative intent. The high frequency of such relapses has generated interest in perioperative therapy. Radiotherapy (RT) in this setting is limited by the technical challenges inherent in abdominal irradiation, optimal definition of fields, diminished performance status, and nutritional state of many patients with gastric cancer.
■A prospective randomized trial from the British Stomach Cancer Group failed to demonstrate a survival benefit for postoperative adjuvant radiation alone, although locoregional failures had decreased from 27% to 10.6%.
■Attempts to improve the efficacy and minimize toxicity with newer RT techniques have been investigated. Sixty patients who underwent curative resection at the National Cancer Institute were randomized to either receive adjuvant intraoperative radiotherapy (IORT) or conventional RT. IORT failed to afford a benefit over conventional therapy in overall survival and remains unavailable to many outside of a clinical trial or specialized center.
■In patients with locally unresectable pancreatic and gastric adenocarcinoma, the Gastrointestinal Tumor Study Group (GITSG) has shown that combined-modality therapy is superior to either RT or chemotherapy alone. On the basis of this concept, combined chemoRT (typically in combination with 5-FU) has been evaluated in both the neoadjuvant (preoperative) and the adjuvant (postoperative) settings.
Perioperative Chemoradiotherapy
Aside from GEJ and high gastric cardia tumors, the available data on the role of neoadjuvant chemoRT for gastric cancer are not conclusive. Although neoadjuvant therapy may reduce the tumor mass in many patients, several randomized, controlled trials have shown that, compared with primary resection, a multimodal approach does not result in a survival benefit in patients with potentially resectable tumors. In contrast, for some patients with locally advanced tumors (i.e., patients in whom complete tumor removal with upfront surgery seems unlikely), neoadjuvant chemoRT may increase the likelihood of complete tumor resection on subsequent surgery. However, predicting those likely to benefit from this approach remains an ongoing research question.
Adjuvant chemoRT has been evaluated in the United States. In a phase 3 Intergroup trial (INT-0116), 556 patients with completely resected stage IB to IV M0 adenocarcinoma of the stomach and GEJ were randomized to receive best supportive care or adjuvant chemotherapy (5-FU and leucovorin) and concurrent radiation therapy (45 Gy). With >6-year median follow-up, median survival was 35 months for the adjuvant chemoRT group as compared to 27 months for the surgery-alone arm (P = 0.006). Both 3-year overall survival (50% vs. 41%; P = 0.006) and relapse-free survival (48% vs. 31%; P < 0.0001) favored adjuvant chemoRT. Although treatment-related mortality was 1% in this study, only 65% of patients completed all therapies as planned and many had inadequate lymph node resections (54% D0). After 10-year median follow-up, persistent benefit in overall survival (HR 1.32; 95% CI 1.10 to 1.60; P = 0.0046) and relapse-free survival (HR 1.51; 95% CI 1.25 to 1.83; P = 0.001) were observed without excess treatment related late toxicities. This study established adjuvant chemoRT as a standard of care for gastric cancer in the United States.
Perioperative Chemotherapy
In Japan, patients who underwent complete surgical resection for stage II and III gastric cancer with D2 lymphadenectomy appeared to benefit from adjuvant S-1, a novel oral fluoropyrimidine. In a randomized controlled trial, patients were randomized to 1 year of monotherapy or surveillance only. The study was closed early after interim analysis confirmed a 3-year overall survival (80% vs. 70%; P = 0.002) and relapse-free survival (72% vs. 60%; P = 0.002) advantage in favor of adjuvant chemotherapy. At 5-year follow-up, the improved overall survival rate (72% vs. 61%) and relapse-free survival rate (65% vs. 53%) persisted. S-1 is approved for adjuvant therapy for gastric cancer in Japan and for advanced gastric cancer in Europe, but it is not commercially available in the United States.
In Europe, focus has been on the role of more potent polychemotherapy regimens in the perioperative setting without RT. The UK Medical Research Council conducted a randomized controlled trial (MAGIC trial) comparing three cycles of pre- and postoperative epirubicin, cisplatin, and 5-FU (ECF) to surgery alone in patients with resectable stage II to IV nonmetastatic gastric cancer; 503 patients were stratified according to surgeon, tumor site, and performance status. Perioperative chemotherapy improved 5-year overall survival (36% vs. 23%; P = 0.009) and reduced local and distant recurrence. There appeared to be significant downstaging by chemotherapy treatment, with more patients deemed by the operating surgeon to have had a “curative” resection (79% vs. 70%; P = 0.03), had smaller tumors (median 3 vs. 5 cm; P < 0.001), had T1/T2 stage tumors (52% vs. 37%; P = 0.002), and had N1/N2 stage disease (84% vs. 71%; P = 0.01). Toxicity was feasible with postoperative complications comparable; however, nearly one-third of patients who began with preoperative chemotherapy did not receive postoperative chemotherapy due to progressive disease, complications, or patient request.
A French multicenter trial also showed a survival benefit for perioperative chemotherapy. Patients with potentially resectable stage II or higher adenocarcinoma of the stomach, GEJ, or distal esophageal (total 224) were randomly assigned to two or three preoperative cycles of cisplatin/5-FU infusion and three or four postoperative cycles of the same regimen versus surgery alone. At a median follow-up of 5.7 years, 5-year OS (38% vs. 24%; HR 0.69; 95% CI 0.50 to 0.95; P = 0.02) and disease-free survival (34% vs. 19%; HR 0.65; 95% CI 0.48 to 0.89; P = 0.003) was improved in the polychemotherapy arm. Curative resection rate was significantly improved with perioperative polychemotherapy (84% vs. 73%; P = 0.04) with similar postoperative morbidity in the two groups. In Europe, perioperative polychemotherapy is considered a standard of care.
Postoperative ChemoRT Versus Perioperative Chemotherapy
There are no randomized controlled trials directly comparing these two standards of care. The ARTIST randomized phase III trial did not show a survival improvement with adjuvant chemoRT compared to adjuvant chemotherapy alone in patients with D2 resected gastric cancer. Patients (n = 458, stage IB to IV M0) were randomly assigned to chemotherapy (capecitabine and cisplatin) or chemoRT (cisplatin/capecitabine followed by capecitabine/radiation [45 Gy] followed by cisplatin/capecitabine). After >4-year follow-up, no significant difference in locoregional recurrences (8.3% in chemo alone vs. 4.8% in chemoRT; P = 0.3533) or distant metastases (24.6% in chemo vs. 20.4% in chemoRT; P = 0.5568) were observed. Treatment completion rate was better than the INT-0116 trial with 75% of patients having completed the planned chemotherapy and 82% the chemoRT. Given that a multivariate analysis showed chemoRT improved 3-year disease-free survival in those with node-positive disease (HR 0.68; 95% CI 0.47 to 0.99; P = 0.047), a subsequent phase III trial (ARTIST-II) study to evaluate the benefit of chemoRT in patients who underwent D2 lymph node dissection with positive lymph nodes has been planned.
CALGB 80101, a US Intergroup study, compared the INT-0116 adjuvant chemoRT versus postoperative ECF before and after chemoRT. Patients (n = 546) with completely resected gastric or GEJ tumors that were ≥T2 or node positive were included. Through a preliminary report, patients receiving ECF had lower rates of diarrhea, mucositis, and grade ≥4 neutropenia. However, the primary endpoint of overall survival was not significantly better with ECF at 3 years (52% vs. 50%). The primary tumor location did not impact treatment outcome.
Unresectable or Metastatic Disease
Primary goals of therapy should focus on improvement in symptoms, delay of disease progression, pain control, nutritional support, and quality of life. Although a role for palliative surgery and RT exist (see previous sections), chemotherapy remains the primary means of palliative treatment in this setting. The most commonly administered chemotherapeutic agents with objective response rates in advanced gastric cancer include mitomycin, antifolates, anthracyclines, fluoropyrimidines, platinums, taxanes, and topoisomerase inhibitors. Monotherapy with a single agent results in a 10% to 30% response rate with mild toxicities (Table 5.3). 5-FU is the most extensively studied agent, producing a 20% response rate. Complete responses with single agents are rare and disease control is relatively brief. Combination chemotherapy provides a better response rate with survival advantage over best supportive care in randomized studies.
Palliative Surgery and Stents
This should be considered in patients with obstruction, bleeding, or pain, despite operative mortalities of 25% to 50%. Gastrojejunostomy bypass surgery alone may provide a twofold increase in mean survival. The selection of patients most likely to benefit from this or other palliative surgical interventions requires further evaluation with prospective studies and multidisciplinary conference discussion.
Plastic and expansile metal stents are associated with successful palliation of obstructive symptoms in more than 85% of patients with tumors in the GEJ and in the cardia.
Palliative Chemotherapy Various combinations of active agents have been reported to improve the response rate (20% to 50%) among patients with advanced gastric carcinoma (Table 5.4
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree