K-ras Detection
The K-ras gene is mutated in 30% to 50% of CRCs, and the detection in stool represents a potentially powerful screening strategy. This is currently an active area of clinical investigation.
PATHOPHYSIOLOGY
More than 90% of CRCs are adenocarcinomas, the focus of this chapter. Other primary cancers of the colon and rectum include Kaposi’s sarcoma, non-Hodgkin’s lymphomas, small cell carcinoma, and carcinoid tumors. Although uncommon, metastases to the large bowel include melanoma, ovarian, and gastric cancer. Anatomic location and symptoms at presentation are the primary differences between right colon, left colon, and rectal adenocarcinomas.
Colon carcinogenesis involves progression from hyperproliferative mucosa to polyp formation, with dysplasia, and transformation to noninvasive lesions and subsequent tumor cells, with invasive and metastatic capabilities. CRC is a unique model of multistep carcinogenesis resulting from the accumulation of multiple genetic alterations. Stage-by-stage molecular analysis has revealed that this progression involves several types of genetic instability, including loss of heterozygosity, with chromosomes 8p, 17p, and 18q representing the most common chromosomal losses. The 17p deletion accounts for loss of p53 function, and 18q contains the tumor-suppressor genes deleted in colon cancer (i.e., DCC) and the gene deleted in pancreatic 4 (i.e., DPC4).
Colon carcinogenesis also occurs as a consequence of defects in the DNA mismatch–repair system. The loss of hMLH1 and hMSH2, predominantly, in sporadic cancers leads to accelerated accumulation of additions or deletions in DNA. This MSI contributes to the loss of growth inhibition mediated by transforming growth factor-β due to a mutation in the type II receptor. Mutations in the APC gene on chromosome 5q21 are responsible for FAP and are involved in cell signaling and in cellular adhesion, with binding of β-catenin. Alterations in the APC gene occur early in tumor progression. Mutations in the proto-oncogene ras family, including K-ras and N-ras, are important for transformation and also are common in early tumor development.
DIAGNOSIS
Signs and Symptoms
■Abdominal pain, typically intermittent, and vague
■Weight loss
■Bowel changes for left-sided colon and rectal cancers, including constipation, decreased stool caliber (pencil stools), and tenesmus
■Early satiety
■Fatigue
■Bowel obstruction, perforation, acute, or chronic bleeding, or liver metastasis, all of which contribute to symptom development
■Unusual presentations include deep venous thrombosis, Streptococcus bovis bacteremia or endocarditis, and nephrotic-range proteinuria
■Clinical findings include iron-deficiency anemia, weight loss, electrolyte abnormalities, and liver enzyme elevations
Diagnostic Evaluation
■Endoscopic studies provide histologic information, potential therapeutic intervention, and overall greater sensitivity, and specificity.
■CEA elevations occur in non–cancer-related conditions, reducing the specificity of CEA measurements alone in the initial detection of colon cancer.
■Basic laboratory studies including complete blood count, electrolytes, liver, and renal function tests, and CT scan of the chest, abdomen, and pelvis with IV contrast are useful in initial cancer diagnosis and staging.
■In colon cancers, CT scan sensitivity for detecting distant metastasis is higher (75% to 87%) than for detecting nodal involvement (45% to 73%) or the extent of local invasion (~50%). CT scanning is very sensitive for detection of malignant pelvic lymph nodes in rectal cancer as any perirectal adenopathy is presumed to be malignant, since benign adenopathy is not typically seen in this area.
■Contrast-enhanced magnetic resonance imaging (MRI) can help determine the status of suspicious lesions in the liver as well as the characteristics (not just size) of perirectal adenopathy.
■PET scanning adds little over conventional imaging in the initial staging and diagnosis of CRC in the absence of abnormalities seen on CT scan.
■Endoscopic rectal ultrasound (EUS) is a valuable tool in the preoperative evaluation of rectal cancer, with high accuracy of determining the extent of the primary tumor (63% to 95%) and perirectal nodal status (63% to 82%).
STAGING
The seventh edition of the American Joint Committee on Cancer Staging for CRC uses the TNM classification system. The Dukes or MAC staging systems are only of historic interest. The tumor designation, or T stage, defines the extent of bowel wall penetration including invasion into the submucosa (T1), muscularis propria (T2), pericolic tissue (T3), visceral peritoneal surface (T4a), or an adjacent organ or other structure (T4b). At least 12 lymph nodes must be sampled for accurate staging and represents an important quality control metric. The number of regional nodes involved varies from 1 to 3 (N1a/b) to 4 or more (N2a/b). N1c includes direct tumor deposits in the subserosa, mesentery, or nonperitonealized pericolic or perirectal tissues without regional nodal metastasis. Metastases confined to one organ or site (M1a) have a better prognosis than metastases confined to the peritoneum or multiple sites (M1b).
PROGNOSIS
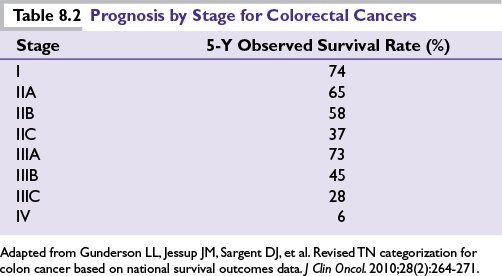
Pathologic staging remains the most important determinant of prognosis (Table 8.2) with similar outcomes for both colon and rectal cancers in the modern era. Other prognostic variables that have been proposed to be associated with an unfavorable outcome include
■Advanced age of patient
■High tumor grade
■High CEA level
■Bowel obstruction or perforation at presentation
■Several biochemical and molecular markers such as elevated thymidylate synthase, p53 mutations, or loss of heterozygosity of chromosome 18q (DCC gene) may be associated with a worse prognosis. MSI caused by a defective DNA mismatch–repair system (e.g., altered MLH1, MSH2; associated with Lynch Syndrome) is associated with an improved outcome for patients with node-negative disease
MANAGEMENT ALGORITHM
Surgery
■For colon cancers, the primary curative intervention requires en bloc extirpation of the involved bowel segment and mesentery, with pericolic and intermediate lymphadenectomy for both staging and therapeutic intent. Negative proximal, distal, and lateral surgical margins are of paramount importance. Laparoscopic techniques adhering to these surgical principles are an acceptable option.
■For rectal cancers, en bloc resection of the primary tumor with negative proximal, distal, and radial margins is critical as well as a sharp dissection of the mesorectum (total mesorectal excision) to optimally reduce local recurrence. The location of the tumor in relation to the anal sphincter is the primary determinant in a low anterior resection (LAR) versus an abdominoperineal resection (APR). The latter generates a permanent colostomy. For highly selected very early-stage rectal cancer cases, transanal endoscopic microsurgery may be a reasonable option.
■Surgical intervention is indicated if polypectomy pathology reveals muscularis mucosal involvement or penetration.
■Surgical palliation may include colostomy or even resection of metastatic disease for symptoms of acute obstruction or persistent bleeding.
Radiation Therapy
■Routine administration of abdominal radiotherapy (RT) is limited by bowel-segment mobility, adjacent small bowel toxicity, previous surgery with adhesion formation, and other medical comorbidities.
■Local control and improved disease-free survival (DFS) have been reported in retrospective series of patients with T4 lesions or perforations, nodal disease, and subtotal resections, who have been treated with 5,000 to 5,400 cGy directed at the primary tumor bed and draining lymph nodes. However, there are no randomized data to support the routine use of RT in the management of colon cancer.
■In contrast, RT is routinely utilized in rectal cancers to reduce local recurrence and improve resectability.
Pivotal Adjuvant Chemotherapy Studies for Colon Cancer
Intergroup 0035
This large Intergroup trial of 5-fluorouracil (5-FU) and levamisole (Lev) is of historic importance because it reported a 41% reduction in the relapse rate and a 33% decrease in overall cancer mortality. This study resulted in the National Institutes of Health consensus panel recommending that 5-FU-based adjuvant therapy be administered to all patients with resected stage III colon cancer.
Intergroup 0089
Intergroup 0089 randomized 3,759 patients with stage II or III disease to one of four therapeutic arms. The results demonstrated that the 5-FU- and leucovorin (LV)-containing schedules (Mayo Clinic and Roswell Park regimens) were equivalent without the need for Lev. A 6-month schedule of the 5-FU and LV was similar to a protracted 12 months of therapy.
The 5-year DFS and overall survival (OS) for each of the four arms in the study were as follows:
■5-FU + Lev for 12 months; DFS = 56%, OS = 63%
■5-FU + high-dose LV (Roswell Park) for 8 months; DFS = 60%, OS = 66%
■5-FU + low-dose LV (Mayo) for 6 months; DFS = 60%, OS = 66%
■5-FU + LV + Lev; DFS = 60%, OS = 67%
X-ACT
Utilization of an oral fluoropyrimidine (capecitabine) was evaluated in patients with stage III disease. Capecitabine (1,250 mg/m2 b.i.d. for 14 days, every 3 weeks) was compared with the Mayo Clinic bolus of 5-FU and LV. The study was designed to demonstrate equivalency, with a primary endpoint of 3-year DFS. The capecitabine arm was noninferior and demonstrated a trend toward superiority in DFS (64% vs. 60%; HR 0.87; 95% CI 0.75 to 1.00; P = 0.0526). Toxicity was improved in all categories except hand–foot syndrome (HFS). A 3-year DFS endpoint was chosen because a retrospective analysis of more than 20,000 patients demonstrated equivalency to the conventional 5-year OS benchmark and serves as an acceptable endpoint.
MOSAIC
In Europe, 2,219 patients with stage II (40%) and III (60%) disease treated with infusional 5-FU with LV modulation versus the same combination with oxaliplatin (FOLFOX4) every 2 weeks for 6 months, demonstrated a 3-year DFS benefit favoring the FOLFOX4 combination over standard 5-FU with LV (78.2% vs. 72.9%; HR 0.77; 95% CI 0.65 to 0.92; P = 0.002). With a median 6-year follow-up, the OS advantage was confirmed in the patients with stage III disease (72.9% vs. 68.7%; HR 0.80; 95% CI 0.65 to 0.97; P = 0.023). No difference in OS was seen in the stage II population. Treatment with FOLFOX4 was well tolerated, with 41% patients having grade 3 and 4 neutropenia, with only 0.7% being associated with fever. Anticipated grade 3 peripheral neuropathy or paresthesias were observed (12%) which almost entirely resolved 2 years later (0.7%).
NSABP C-07
The addition of oxaliplatin to three cycles of adjuvant Roswell Park 5-FU with LV (FLOX) was evaluated in 2,407 stage II (30%) and III (70%) patients. The combination improved 3-year DFS (76.1% vs. 71.8%; HR 0.80; 95% CI 0.69 to 0.93; P = 0.003). Grade 3 diarrhea (38%) and peripheral neuropathy (8%) were significantly worse with FLOX without any difference in treatment-related mortality. MOSAIC and C-07 established doublet adjuvant chemotherapy with fluoropyrimidine and oxaliplatin as a standard of care.
Adjuvant Irinotecan
Unlike oxaliplatin, at least three studies failed to confirm a benefit for the use of adjuvant irinotecan. CALGB 89803 was a study of irinotecan with bolus 5-FU and LV (IFL) versus weekly 5-FU in patients with stage III disease. Increased grade 3 and 4 neutropenia and early deaths were observed in the experimental arm, and a higher number of patients withdrew from the study. Overall, IFL was not better than the 5-FU and LV arm. The two European studies (PETACC-3 and ACCORD) together randomized over 3,500 patients to infusional 5-FU with or without irinotecan. Both studies failed to reach their primary endpoint of 3-year DFS, although toxicities were less than in the IFL study. The use of irinotecan is not recommended in the adjuvant setting.
Adjuvant Biologics
Both cetuximab (cmab) and bevacizumab (bev) are biologic-targeted agents (see the metastatic CRC section) that have been shown to improve outcomes when combined with chemotherapy in metastatic CRC and have been definitively tested in the adjuvant setting.
Intergroup 0147 tested whether the addition of cmab to standard mFOLFOX6 adjuvant chemotherapy for resected stage III colon cancer improved outcomes. The protocol was amended to allow only patients with wild-type K-ras tumors to be eligible. The study terminated early after a second interim analysis. Results demonstrated no benefit when adding cmab. Three-year DFS for patients with wild-type K-ras was 71.5% with mFOLFOX plus cmab and 74.6% with mFOLFOX alone (HR 1.21; 95% CI 0.98 to 1.49; P = 0.08), suggesting a trend toward harm. There were no subgroups that benefitted from cmab, with increased toxicity and greater detrimental differences in all outcomes in patients aged ≥70.
The addition of bev to mFOLFOX6 was tested in NSABP C-08. This randomized phase III trial assessed DFS in stage II (25%) and III patients. Bev was administered for the duration of the 6 months of chemotherapy and then for an additional 6 months beyond (total of 1 year of biologic therapy). mFOLFOX plus bev did not significantly improve 3-year DFS compared to mFOLFOX (77.4% vs. 75.5%; HR 0.89; 95% CI 0.76 to 1.04; P = 0.15). However, survival curve analysis suggested a time-dependent improvement in DFS with maximal separation of the curves occurring at 15 months, which correlated with 1 year of bev treatment followed by 3 months off drug. This benefit disappears with time. No OS benefit, unexpected toxicity, or difference in patterns of relapse was seen.
The AVANT trial also tested bev in a three-arm study that randomized 3,451 patients with high-risk stage II (17%) or stage III colon cancer to either FOLFOX4, FOLFOX4 plus bev, or XELOX plus bev. The 3-year DFS was not significantly different between the groups with 5-year OS hazard ratio for FOLFOX 4 plus bev versus FOLFOX4 (HR 1.27; 95% CI 1.03 to 1.57; P = 0.02), and XELOX plus bev versus FOLFOX4 (HR 1.15; 95% CI 0.93 to 1.42; P = 0.21) suggesting a potential detriment.
Adjuvant Chemotherapy Regimens for Colon Cancer
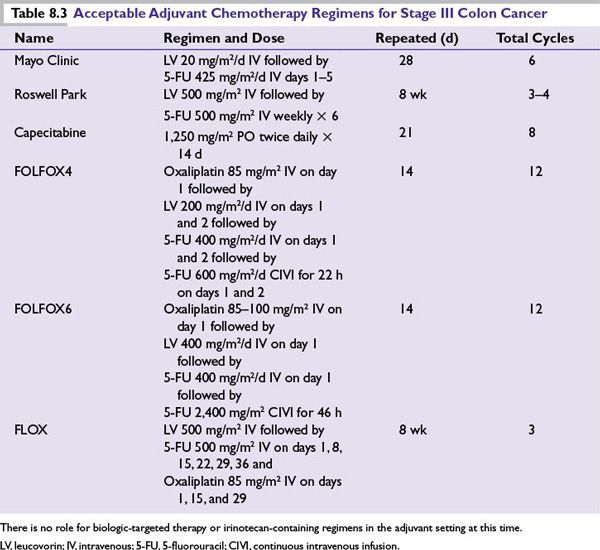
Based on these studies, 6 months of adjuvant chemotherapy is recommended for patients with stage III colon cancer. Several acceptable options exist (Table 8.3), with combination regimens offering increased efficacy and toxicity. The use of irinotecan or biologic-targeted therapies in the adjuvant setting is not recommended. Adjuvant chemotherapy should be started within 8 weeks of surgery, if at all possible, with data supporting a delay beyond 2 months may compromise the effectiveness of adjuvant treatment.
Fluoropyrimidines
Reasonable options include 5-FU with LV via the Mayo Clinic or Roswell Park regimen or capecitabine. The toxicity profile of the regimens differs. Myelosuppression and oral mucositis are more common with the daily Mayo Clinic regimen, whereas diarrhea may be more severe with the weekly Roswell Park schedule. Cryotherapy with ice held in the mouth during the 5-FU infusion may help lessen the mucositis associated with the therapy. HFS and diarrhea are primary toxicities of capecitabine.
Oxaliplatin Combinations
Increased efficacy as well as toxicity is seen with the addition of oxaliplatin to either bolus or infusional 5-FU and LV. FOLFOX6 represents a modification to FOLFOX4, which omits the day 2 bolus 5-FU and LV and gives more continuously infused 5-FU over 46 hours, and appears to have activity equivalent to that of FOLFOX4 in the advanced disease setting. It has been incorporated into numerous adjuvant clinical trials given the improved ease of administration.
Adjuvant Chemotherapy for Stage II Colon Cancer
Despite the 75% 5-year survival with surgery alone, some patients with stage II disease have a higher risk of relapse, with outcomes being similar to those of node-positive patients. Adjuvant chemotherapy provides up to 33% relative risk reduction in mortality, resulting in an absolute treatment benefit of approximately 5%.
Several analyses have reported varying outcomes in patients with stage II disease who received adjuvant treatment:
■The National Surgical Adjuvant Breast and Bowel Project (NSABP) summary of protocols (C-01 to C-04) of 1,565 patients with stage II disease reported a 32% relative reduction in mortality (cumulative odds, 0.68; 95% CI 0.50 to 0.92; P = 0.01). This reduction in mortality translated into an absolute survival advantage of 5%.
■A meta-analysis by Erlichman et al. detected a nonsignificant 2% benefit (82% vs. 80%; P = 0.217) in 1,020 patients with high-risk T3 and T4 cancer treated with 5-FU and LV for 5 consecutive days.
■Schrag et al. reviewed Medicare claims for chemotherapy within the Surveillance, Epidemiology, and End Results (SEER) database and identified 3,700 patients with resected stage II disease among whom 31% received adjuvant treatment. No survival benefit was detected with 5-FU compared to surgery alone (74% vs. 72%) even with patients considered to be at high risk because of obstruction, perforation, or T4 lesions.
■The Quasar Collaborative Group study reported an OS benefit of 3.6% in 3,239 patients (91% Dukes B colon cancer) prospectively randomized to chemotherapy versus surgery alone. With a median follow-up of 5.5 years, the risk of recurrence (HR 0.78; 95% CI 0.67 to 0.91; P = 0.001) and death (HR 0.82; 95% CI 0.70 to 0.95; P = 0.008) favored 5-FU and LV chemotherapy.
■In the MOSAIC study, FOLFOX4 chemotherapy showed nonsignificant benefits in DFS over 5-FU and LV in patients with stage II disease (86.6% vs. 83.9%; HR 0.82; 95% CI 0.57 to 1.17).
■The American Society of Clinical Oncology Panel concluded that the routine use of adjuvant chemotherapy for patients with stage II disease could not be recommended. A review of 37 randomized controlled trials and 11 meta-analyses found no evidence of a statistically significant survival benefit with postoperative treatment of stage II patients. However, treatment should be considered for specific subsets of patients (e.g., T4 lesions, perforation, poorly differentiated histology, or inadequately sampled nodes), and patient input is critical.
■For stage II patients without high-risk features, molecular analysis can provide improved recurrence risk determination.
•
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree