Diagnosis and Distinguishing between the MPNs
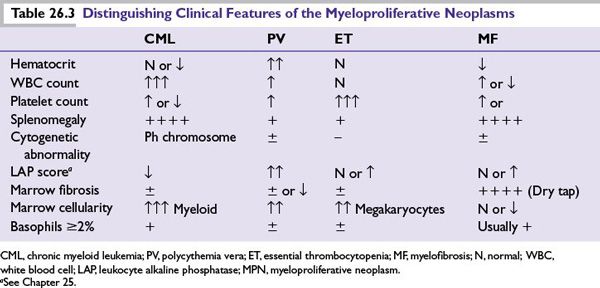
The clinical presentation of MPNs can be with incidentally noted abnormal blood counts with patterns that vary depending on the particular MPN (Table 26.3). Distinctive clinical features relate to these lineage changes and splenomegaly.
■Increased red blood cell (RBC) mass and thus viscosity in PV can produce symptoms such as headaches, vertigo, tinnitus, and blurred vision. Another characteristic of PV in some patients is pruritus (histamine release) aggravated by hot water.
■Increased number of abnormal platelets in ET can cause arterial thrombotic events such as cerebrovascular ischemia, digital ischemia/erythromelalgia, and spontaneous abortions.
■Anemia in patients with MF may cause fatigue and shortness of breath, and splenomegaly can cause abdominal discomfort or early satiety. Hypermetabolic symptoms such as weight loss and sweating can be seen in MF but also in the other MPNs.
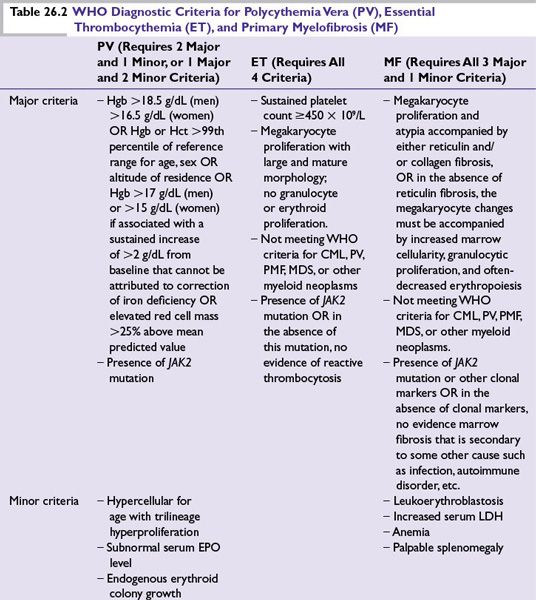
As outlined earlier, there is overlap in the molecular underpinnings of MPNs, and thus, not surprisingly, in clinical behaviors. Nonetheless, the various MPN classifications do have differing risks and prognoses; thus, the diagnostic approach prioritizes not mistakenly classifying an MPN into a category with less urgent prognostic or treatment implications. Accordingly, ET is diagnosed after excluding PV, and MF is diagnosed after excluding PV and ET (because marrow fibrosis can be a sequela of the other MPNs with their greater thrombo-hemorrhagic implications). CML should be ruled out by performing a fluorescence in situ hybridization (FISH) analysis for BCR-ABL in JAK2 mutation-negative thrombocytosis or bone marrow fibrosis. Even with a positive JAK2 mutation or other clinical and peripheral blood observations to favor a particular MPN classification, bone marrow biopsy with cytogenetic analysis is recommended, to not miss a diagnosis of CML or MDS with accompanying prognostic and treatment implications. Platelet function tests or bleeding times are of little use in diagnosing or in guiding the management of MPNs.
PROGNOSIS
Median Survivals
■Patients with PV have a median survival of 1.5 to 13 years. In a recent multicountry prospective study of 1,638 patients with PV, the 5-year event-free survival was 82%, with a relatively low risk of death from cardiovascular disease and a high risk of death from noncardiovascular causes (mainly hematologic transformations).
■Patients with ET have a median survival of more than 10 years.
■Patients with MF have a median survival between 3 and 5 years.
Rate of Transformation to Acute Leukemia
■The estimated incidence of acute leukemia in 1,638 patients with PV prospectively followed in the ECLAP study was 1.3%, with an estimated annual incidence of 0.5 per 100,000 per year. Older age and exposure to P32, busulfan, or pipobroman were independent risk factors.
■The cumulative rate of transformation for patients with ET is 2% to 4%, respectively, at 10 and 20 years from diagnosis.
■The cumulative rate of transformation for patients with MF is 10% at 10 years (please also see discussions on treatment regarding transformation risk).
Transformation of PV or ET into MF
Both PV and ET may progress to post-PV MF or post-ET MF, previously referred to as the spent phase, which clinically resembles primary MF and is characterized by progressive cytopenias, splenomegaly, and marrow fibrosis. The cumulative rate of transformation is 5% and 10% at 10 years to 20 years, respectively, for ET and 10% to 20% for the same time line for PV.
Risk Factors for Thrombosis
In two prospective studies, the ECLAP study and the MRC-PT1, the cumulative rate of cardiovascular events in patients with PV ranged from 2.5% to 5% per patient-year and from 1.9% to 3% per patient-year for patients with ET. Arterial thrombosis accounts for 60% to 70% of the events, and is the major cause of death.
■In PV, older age (>60), a hematocrit ≥45%, and a previous history of thrombosis are risk factors. Surgery should be avoided in patients until a hematocrit <45% has been maintained for more than 2 months.
■In ET, age over 60 years and the presence of other cardiovascular risk factors (e.g., smoking and previous thrombosis) increase the risk for thrombosis.
In ET, an association between platelet count and thrombosis has not been established, but platelet cytoreduction on treatment with hydroxyurea (HU) has been associated with a reduced risk.
Risk Factors for Hemorrhage
■In ET, a platelet count >2 × 106/μL is a risk factor for hemorrhage (please also see recommendations regarding treatment).
TREATMENT
As a general principle, treatment for PV, ET, MF, or overlaps thereof is aimed at (i) alleviating the particular symptoms present in the individual patient (e.g., symptoms from splenomegaly, or symptoms from cytopenia) and (ii) anticipating and preventing potential life-threatening complications such as thrombosis or hemorrhage. Bone marrow transplantation is a potentially curative option that should be considered for some patients with MF. Following is a definition of risk categories and recommended treatments, with an overview provided in Table 26.4.
Polycythemia Vera
■Low risk: Age <60 years with no personal history of vascular events and who do not have additional risk factors for cardiovascular disease. Recommended treatment: phlebotomy alone with or without low-dose aspirin.
■Intermediate risk: Age <60 years with no personal history of vascular events and who do have additional cardiovascular risk factors. Recommended treatment: phlebotomy alone is adequate therapy; use of low-dose aspirin is encouraged.
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree