Indications for Genetic Testing
Genetic testing is available commercially (Myriad Genetics). All patients should undergo genetic counseling before undergoing the test. There are three possible outcomes of genetic testing for the BRCA mutations: positive, variant of uncertain significance, or negative. A negative result indicates no increased risk of breast cancer due to a germ-line mutation of the BRCA1/2 genes. A variant of uncertain significance (indeterminate) test result indicates that no conclusive evidence exists to indicate that the mutation does or does not carry an increased risk of the development of breast cancer due to an inherited genetic mutation. A positive result indicates that there exists a mutation in the BRCA1 or 2 genes that have been associated with an inherited risk of developing breast cancer.
As per NCCN guidelines (accessed January 2013), patients with breast cancer with one or more of the following should undergo further genetic risk evaluation:
■Early-age onset breast cancer
■Triple negative breast cancer (ER–, PR–, HER-2/neu-)
■≥2 breast primaries in a single individual or two different individuals from the same side of the family
■≥1 close blood relative (first-, second-, or third-degree relative) with breast cancer ≤50 years of age
■≥2 close blood relatives with breast cancer and/or pancreatic cancer at any age
■Population at increased risk (i.e., women of Ashkenazi Jewish descent)
■Male breast cancer
Management of Patients with Positive BRCA Test
Management recommendations for patients with a known genetic mutation are highly individualized and should be made by an expert. Recommendations include the following:
■Breast self-examination training and education starting at age 18.
■Clinical breast examination every 6 to 12 months, starting at age 25.
■Annual mammogram and breast magnetic resonance imaging (MRI) starting at age 25 or earlier based on family history.
■Discuss options of bilateral prophylactic mastectomy on a case-by-case basis, since it could prevent breast cancer in 90% to 100%.
■Recommend bilateral salpingo-oophorectomy (BSO) ideally between the ages of 35 and 40 or after completion of child bearing. BSO alone will reduce breast cancer risk by 50% and prevents ovarian cancer by 95%.
■Patients who defer BSO may consider concurrent trans-vaginal ultrasound with CA-125 lab draw every 6 months starting at the age of 30 or 5 to 10 years prior to the earliest age of ovarian cancer in the family.
CHEMOPREVENTION
Risk Assessment
■The Gail model (http://www.nci.nih.gov) is a statistical model that calculates a woman’s absolute risk of developing breast cancer by using the following criteria: age, age at menarche, age at first live birth, number of previous biopsies, history of atypical ductal hyperplasia (ADH), and number of first-degree relatives with breast cancer. This model is not intended to be used in patients with an existing history of invasive cancer, DCIS, or lobular carcinoma in situ (LCIS). The Gail model underestimates the risk of breast cancer in a person with hereditary breast cancer.
Prevention Studies
The National Surgical Adjuvant Breast and Bowel Project
■The National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 study showed a 49% reduction in the incidence of invasive breast cancer in high-risk subjects who took tamoxifen at a dose of 20 mg daily for 5 years.
■Women eligible for this trial were at least 35 years old and were assessed to have an absolute risk of at least 1.67% over the period of 5 years using the Gail model or a pathologic diagnosis of LCIS.
■Use of tamoxifen for breast cancer should be individualized, and must be considered after weighing the risk:benefit ratio for each patient.
■Women with a life expectancy of ≥10 years and no diagnosis/history of breast cancer considered at increased risk of breast cancer should receive individualized counseling to decrease breast cancer risk.
NSABP P-2: Study of Tamoxifen and Raloxifene
In the NSABP P-2 study, tamoxifen 20 mg daily was compared with raloxifene 60 mg daily in postmenopausal women with high risk of developing breast cancer (Gail risk model 1.66%). The results of the study revealed that raloxifene was equivalent to tamoxifen in preventing invasive breast cancer (about a 50% reduction). Raloxifene did not reduce the risk of DCIS or LCIS unlike tamoxifen.
Raloxifene has a better side effect profile, which resulted in a lower incidence of uterine hyperplasia, hysterectomy, cataracts, and a lower rate of thromboembolic events. In postmenopausal patients, due to equal efficacy and better side effect profile, raloxifene 60 mg daily could be used instead of tamoxifen for breast cancer prevention.
Aromatase Inhibitors for Risk Reduction
The Arimidex, Tamoxifen alone, or in Combination Trial (ATAC Trial) showed a nonsignificant reduction in contralateral breast cancers in women treated with anastrozole alone when compared with tamoxifen (P = 0.62). A significant reduction (P = 0.04) was noted in contralateral breast cancers in a subset of women with hormone receptor-positive first cancers.
The Breast International Group (BIG) 1-98 trial compared postmenopausal women with early-stage breast cancer to those who underwent 5 years of therapy and found that risk of breast cancer recurrence was lower in women in the letrozole arm when compared to the tamoxifen arm.
The MAP.3 trial evaluated the role of exemestane in a risk reduction setting, randomizing women to either exemestane or placebo. A median follow-up of 3 years showed that exemestane reduced the relative incidence of breast cancers by 65% when compared to placebo. It was not associated with any significant serious side effects and only minimal changes in quality of life.
Summary
In premenopausal women with increased risk of breast cancer as per the Gail model it is reasonable to recommend tamoxifen 20 mg daily for 5 years. In postmenopausal women raloxifene and tamoxifen are equally effective, but raloxifene has been shown to have less side effects. Exemestane can also be considered; however, the FDA has not approved exemestane in this setting at this time. Any risk reduction approach should be carefully decided after a detailed risk versus benefit discussion with the patient.
There are only limited data for chemoprevention in patients with BRCA mutation. One study showed that tamoxifencan reduces the risk by 62% compared to placebo; however, tamoxifen use was not associated with reduction in risk in those patients with a BRCA1 mutation. Clinical trials are addressing the role of AIs in breast cancer prevention in mutation carriers.
Screening Mammograms and MRI
■The National Cancer Institute, American Cancer Society, and American College of Radiology all recommend mammography for women aged 40 years and older.
■Women aged 40 years and older at an average risk of breast cancer should have mammograms every 1 to 2 years.
■Women who are at higher than average risk of breast cancer (those with a family history of breast cancer or with either the BRCA1 or the BRCA2 gene) should discuss with their health care providers about whether to have mammograms before the age of 40 and how often to have them.
■Mammograms should be continued regardless of a woman’s age, as long as she does not have serious, chronic health problems such as congestive heart failure, end-stage renal disease, chronic obstructive pulmonary disease, and moderate to severe dementia. Age alone should not be the reason to stop having regular mammograms. Women with serious health problems or short life expectancies should discuss with their doctors whether to continue having mammograms.
■Screening mammograms help reduce death in patients between the ages of 40 and 70 years.
■Potential harm of screening includes false-negative and false-positive results, over diagnosis and overtreatment.
■There is considerable controversy among various experts regarding the risk and benefit screening mammogram, especially women between 40 and 50 years.
Digital Mammography
The diagnostic superiority of digital mammography was demonstrated in the Digital Mammographic Imaging Screening Trial (DMIST). This study concluded that pre- or perimenopausal women under the age of 50, or women at any age with dense breasts, had a more accurate detection of breast cancer with the digital mammogram. The amount of radiation exposure in digital mammograms is less than film mammograms.
Magnetic Resonance Imaging
Breast MRI has been shown to have a higher sensitivity than mammography. Specificity is however lower, which will result in more false positives and therefore more biopsies. In a high-risk population, MRI and mammogram (92.7%) have a higher sensitivity than mammogram and ultrasound combined (52%). In high-risk women, breast MRI is cost effective, specifically those women with BRCA gene mutations (along with untested first-degree relatives) and women whose lifetime risk of breast cancer exceeds 20%. Patients need to be carefully selected for additional screening with MRI. MRI is recommended in patients with prior radiation therapy who are ≥25 years of age, and women with a genetic predisposition for breast cancer starting at the age of 25.
CLINICAL FEATURES OF BREAST CANCER
Clinical features may include a breast lump, skin thickening or alteration, peau d’orange, dimpling of the skin, nipple inversion or crusting (Paget disease), unilateral nipple discharge, and new onset pain. Patients may instead present with signs and symptoms of metastatic disease.
DIAGNOSIS
1.History and physical examination
2.Bilateral mammogram (80% to 90% accuracy)
3.Biopsy: Any distinct mass should be considered for a biopsy, even if the mammograms are negative. The standard methods of diagnosis for palpable lesions are
–Core-needle biopsy
–Incisional or excisional biopsy
The options in nonpalpable breast lesions are
–Ultrasound-guided core-needle biopsy
–Stereotactic core-needle biopsy under mammographic localization
–Needle localization under mammography, followed by surgical excision
–MRI-guided biopsy
4.Laboratory studies
–Complete blood count, liver function tests, and alkaline phosphatase level.
–Routine use of breast cancer markers such as CA 27:29 and 15:3 is not recommended.
5.Pathology and special studies
–Histology and diagnosis (invasive vs. in situ)
–Pathologic grade of the tumor
–Tumor involvement of the margin
–Tumor size
–Lymphovascular invasion
6.Estrogen receptor/progesterone receptor (ER/PR) status should be done in all tumors (both invasive and noninvasive) and biopsies of metastatic or recurrent (patients those who relapsed) lesions.
–As per the ASCO/CAP guidelines (2010) ER/PR is considered as positive if 1% of tumor cell nuclei are immunoreactive.
7.HER-2/neu- testing (as per ASCO/CAP Guidelines 2013)
–Positive for HER-2/neu- is either IHC 3 + (defined as uniform intense membrane staining of more than 30% of invasive tumor cells) or FISH amplified (ratio of HER-2/neu- to CEP17 of more than 2.0 or average HER-2/neu- gene copy number more than six signals/nucleus for those test systems without an internal control probe).
–Equivocal for HER-2/neu- is defined as either IHC 2 + or FISH ratio of <2 or average HER-2/neu- gene copy number of 4 to 6 signals/nucleus for test systems without an internal control probe.
–Negative for HER-2/neu- is defined as either IHC 0–1 + or FISH ratio of less than <2 with an average HER-2/neu- gene copy number of less than 4 signals/nucleus for test systems without an internal control probe.
8.Indices of proliferation (e.g., mitotic index, Ki-67, or S phase) can be helpful. Ki-67 can be helpful in distinguishing luminal A versus B in ER/PR-positive lesions.
9.Radiographic studies are performed on the basis of the findings of the history and physical examination and blood tests.
Appropriate imaging studies such as CAT scan, ultrasound, MRI, or CT/PET scan can be considered as per the clinical indications. They are not routinely recommended for all patients.
10.Breast MRI is indicated in the following (American College of Radiology Guidelines):
–Evaluating the extent of disease in known cancer patients
•Multifocal and multicentric disease
•Pectoralis and chest wall involvement
•Contralateral breast cancer
–Evaluating response to neoadjuvant chemotherapy
–Axillary adenopathy, primary unknown
–Postlumpectomy for residual disease (close or positive margins)
–Suspected recurrence of breast cancer
•Inconclusive mammographic/clinical findings
•Reconstruction with tissue flaps or implants
–Lesion characterization
•Inconclusive findings on mammogram, ultrasound, physical examination
11.Positron emission tomography (PET) scan. PET scans are of low yield in patients with early (stage I and II) breast cancer. CT/PET scan may be useful in patients with locally advanced or metastatic breast cancer.
PATHOLOGY
Infiltrating or invasive ductal cancer is the most common breast cancer histologic type and comprises 70% to 80% of all cases (Table 12.2).
STAGING OF BREAST CANCER
For staging of breast cancer the American Joint Committee on Cancer (AJCC) manual, seventh edition, should be followed.
Prognostic Factors
Anatomic features such as tumor size and lymph node status are important prognostic features. But biologic features of the tumor are equally important or possibly even more important than anatomic features.
1.Number of positive axillary lymph nodes
–This is an important prognostic indicator. Prognosis is worse with increasing number of lymph nodes.
2.Tumor size
–In general, tumors smaller than 1 cm have a good prognosis in patients without lymph node involvement.
3.Histologic or nuclear grade
–Patients with poorly differentiated histology and high nuclear grade have a worse prognosis than others.
–Scarff-Bloom-Richardson grading system and Fisher nuclear grade are commonly used systems. The modified Scarff-Bloom-Richardson grading system assigns a score (1 to 3 points) for features such as size, mitosis, and tubule formation. These scores are added and tumors are labeled low grade (3 to 5 points), intermediate grade (6 to 7 points), or high grade (8 to 9 points).
4.ER/PR status
–ER- and/or PR-positive tumors have better prognosis and these patients are eligible to receive endocrine therapy.
5.Histologic tumor type
–Prognoses of infiltrating ductal and lobular carcinoma are similar.
–Mucinous (colloid) and tubular histologies have better prognosis.
–Inflammatory breast cancer is one of the most aggressive forms of breast cancer.
6.HER-2/neu- expression
–HER-2/neu- overexpression is a poor prognostic marker and patients with HER-2/neu- overexpression are candidates for HER-2/neu-targeted therapies. Availability of effective HER-2/neu-targeted therapies has revolutionized the treatment and outcome of HER-2/neu-positive breast cancer. Because of targeted therapies, for all practical purposes, HER-2/neu- positivity can be considered as a good prognostic feature now. It is important to remember that patients with a FISH ratio between 2.0 and 2.2 were considered as HER-2/neu- positive and were eligible for treatment in the early adjuvant trastuzumab trials. So patients with FISH ratio more than 2 should be considered for treatment with HER-2/neu-targeted drugs, especially trastuzumab in adjuvant settings.
7.Gene expression profiles
•Oncotype DX is a diagnostic genomic assay based on RT-PCR on paraffin-embedded tissue (Fig. 12.1). This assay was initially developed to quantify the likelihood of cancer recurrence in women with newly diagnosed, stage I or II, node-negative, ER-positive breast cancer. Patients are divided into low-risk, intermediate-risk, and high-risk groups on the basis of the expression of a panel of 21 genes. The recurrence score determined by this assay is found to be a better predictor of outcome than standard measures such as age, tumor size, and tumor grade. Studies have validated the role of Oncotype DX patients with node-positive and ER-positive tumors and it can be used in selected settings. It is being studied in DCIS also.
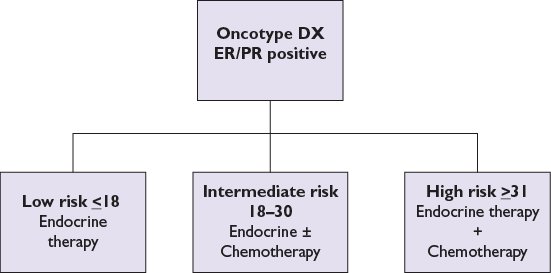
FIGURE 12.1 Oncotype DX assay.
MammaPrint is a DNA microarray assay of 70 genes designed to predict the risk of recurrence of early-stage breast cancer. In February 2007, the FDA approved the use of MammaPrint in patients less than the age of 61, with a tumor size less than 5 cm and lymph node negative.
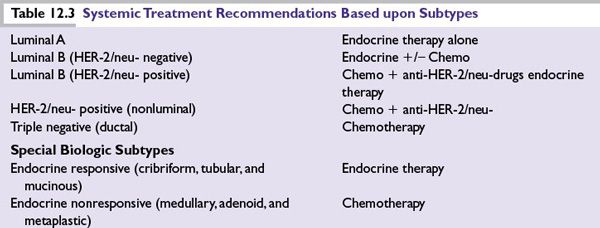
Several distinct types of breast cancer are identified by gene expression studies. They differ markedly in prognosis and in the therapeutic targets they express (Table 12.3).
■Luminal subtypes: Luminal A and luminal B, which express genes associated with luminal epithelial cells of normal breast tissue and overlap with ER-positive breast cancers defined by clinical assays. The luminal A subtype amounts to about 40% of cancers and they have the best prognosis. About 20% of breast cancers are of luminal B subtype and they have worse prognosis compared to luminal A.
■HER-2/neu-enriched subtype (previously the HER-2/neu-positive/ER-negative subtype): The HER-2/neu-enriched subtype comprises the majority of clinically HER-2/neu-positive breast cancers. It accounts for 10% to 15% of breast cancers. Not all HER-2/neu-positive tumors are HER-2/neu-enriched. About half of clinical HER-2/neu-positive breast cancers are HER-2/neu- enriched; the other half can include any molecular subtype including HER-2/neu-positive luminal subtypes.
■ER-negative subtypes: There are several ER-negative subtypes characterized by low expression of hormone receptor-related genes. These include the basal-like, claudin-low, interferon-rich, androgen receptor, and normal-like subtypes. They are mostly triple negative breast cancer and they have a more aggressive clinical course.
MANAGEMENT
High-Risk Lesions
Patients with high-risk lesions may be eligible for breast cancer prevention studies. Tamoxifen and raloxifene are two FDA-approved drugs for breast cancer prevention in high-risk settings. As per the MAP.3 study exemestane was found to be effective in breast cancer prevention.
Atypical Ductal Hyperplasia
■There is a four- to fivefold increase in the risk of developing breast cancer in patients with ADH.
■There is wide variation in the criteria used in the diagnosis of ADH.
■ADH is managed by close follow-up of patients.
■Clinical breast examination and mammogram are the preferred screening methods.
■Tamoxifen 20 mg PO for 5 years: The NSABP P-1 study showed 86% reduction in the risk of developing invasive breast cancer in patients who received tamoxifen.
■The NSABP P-2 study showed similar efficacy for raloxifene 60 mg daily for 5 years, but with fewer adverse effects. Hence, in postmenopausal patients, raloxifene could be considered as the preferred treatment option.
Lobular Carcinoma In Situ
■LCIS is not considered a form of cancer, but a marker of increased risk of developing invasive breast cancer.
■It is usually multicentric and bilateral.
■There is a 21% chance of developing breast cancer in patients within 15 years of developing LCIS.
■It is managed by close follow-up of patients.
■
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree