Value of MMC in the Combined-Modality Regimen
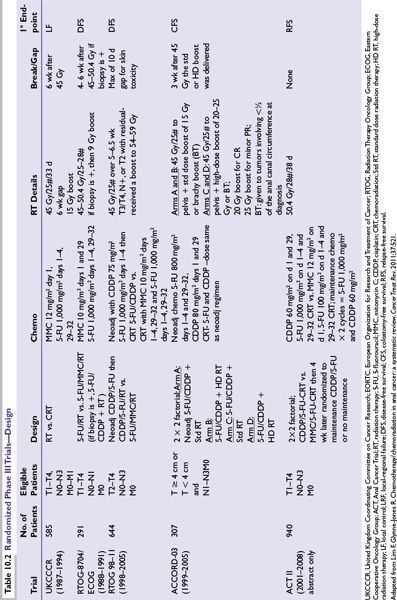
Due to the hematologic toxicity of MMC in CMT, two prospective phase III trials (RTOG/ECOG 8704 and RTOG 98-11) have evaluated the importance of MMC (Tables 10.2 and 10.3).
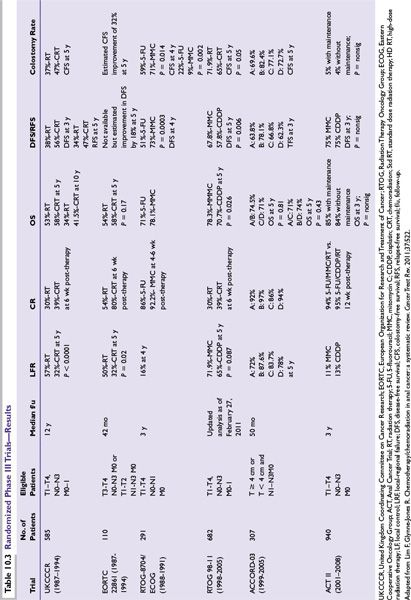
RTOG/ECOG 8704 was the first randomized trial to evaluate prospectively the importance of MMC in CMT and compared 5-FU and MMC with RT (standard arm) versus 5-FU and RT (experimental arm). The primary endpoint was DFS. At 4 years, DFS was 73% for 5-FU and MMC compared to 51% for 5-FU. Colostomy rate was 22% with 5-FU vs. 9% with 5-FU, MMC (P = 0.002). Despite the higher toxicity of MMC, the authors concluded that MMC was still the preferred regimen, given the higher DFS and lower colostomy rate.
RTOG 98-11 was the second randomized trial to evaluate prospectively the role of MMC in CMT and compared 5-FU and MMC with concurrent radiotherapy (standard arm) versus induction 5-FU and CDDP followed by concurrent 5-FU and CDDP with radiotherapy (experimental arm). The primary endpoint was again DFS. With a median of 2.51 years, the initial report revealed no statistically significant difference in 5-year DFS (60% MMC vs. 54% CDDP), 5-year OS (75% MMC vs. 70% CDDP), 5-year local-regional control rates (25% MMC vs. 33% CDDP) or 5-year distant metastasis (DM) rates (15% MMC vs. 19% CDDP). The cumulative colostomy rate proved to be statistically significantly higher with CDDP, 19% vs. MMC, 10%.
However, the updated RTOG 98-11 examined the long-term impact of treatment on survival (DFS, OS, CFS), as well as colostomy failure (CF), and locoregional failure (LRF), and DM. With longer follow-up, 5-FU/MMC regimen produced statistically significant improvement in DFS and OS for RT + 5-FU/MMC vs. RT + 5-FU/CDDP (5-year DFS; 67.8% vs. 57.8%; P = 0.006; 5-year OS, 78.3% vs. 70.7%; P = 0.026). There was a trend toward statistical significance for CFS, LRF, and CF. Thus, the authors conclude that RT + 5-FU/MMC remains the preferred standard of care.
ACT III (abstract form only) addressed two questions: (1) whether substituting CDDP for MMC improves the complete response rate and (2) whether two cycles of maintenance chemotherapy (5-FU/CDDP) reduce recurrences. The randomization employed a 2 × 2 factorial, and patients were randomized to 5-FU/MMC with concurrent radiotherapy (standard arm) or CDDP/5-FU with concurrent radiotherapy. Patients were then randomized to receive either two cycles of maintenance chemotherapy (CDDP/5-FU) or no maintenance therapy. At a median follow-up of 3 years, the complete response rate was 94% for MMC and 95% for CDDP (P = 0.53). No statistically significant differences in terms of recurrence-free survival and overall survival were noted between the maintenance and the no maintenance arms. The authors again concluded that 5-FU/MMC with RT should remain the standard of care.
The data from ACT III and the updated RTOG 98-11 may seem to contradict one another: the CDDP arm in RTOG 98-11 appears to have a detrimental effect on DFS and OS, whereas the ACT III shows that CDDP may be at least equivalent to that of MMC. However, the trial designs were quite different. RTOG employed the use of neoadjuvant chemotherapy prior to the start of concurrent chemoradiotherapy. The prolongation of the overall treatment time may account for the inferior results of the CDDP arm. However, given the current data available, 5-FU/MMC should remain the standard of care.
Toxicity of Combined-Modality Therapy
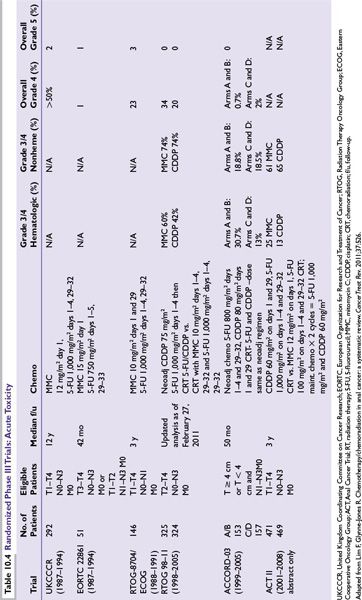
Toxicity from CMT can be divided into acute and late effects. A variety of toxicity criteria have been employed in the different randomized trials, which makes analysis difficult (Table 10.4).
Acute Toxicity
Patients typically experience moderate to severe acute toxicities from the combination of both chemotherapy (5-FU and MMC) and radiation therapy. Side effects include nonhematologic toxicities (nausea/vomiting, abdominal pain, increased frequency of stool, diarrhea, skin irritation, fatigue, weight loss) and hematologic toxicities (neutropenia, thrombocytopenia, anemia).
Toxic deaths from CMT have ranged from 0% to 5%). In the UKCCCR study, 6/116 (2%) experienced toxic death, mostly due to septicemia. The EORTC trial reported on 1 toxic death out 110 patients. In the RTOG 8704 study, four patients (3%) experienced death in the MMC arm. More recently, there were no reported toxic deaths in both RTOG 98-11 and ACT II trials. The ACCORD 03 trial, a four-arm randomized trial, showed similar toxic deaths across all four arms (A = 1 [1%], B = 2 [2.6%], C = 3[3%], D = 1[1%]). No patient required an APR for acute toxicity in any of the arms during the induction phase or the concurrent chemoradiation phase.
Late Toxicity
Late effects have not been well documented within the randomized trials. Part of the challenge in evaluating late effects is the differing toxicity scales used in the various trials. Early toxicity criteria used in the randomized trials did not allow for characterizing radiation-induced side effects.
Both the early EORTC and UKCCCR did not demonstrate a difference in long-term complications between those receiving CMT vs. RT alone. RTOG 8704 similarly revealed no significance difference in long-term toxicity between RT-5-FU and RT and 5-FU/MMC, although two patients from each arm required stoma secondary to RT-related complications. In the RTOG 9811 update, the most common types of late grade 3 or 4 toxicity included skin, small/large intestine, subcutaneous tissue, or other. There did not appear to be a difference between the MMC or CDDP arms for grade 3/4 toxicity (13.1% vs. 10.7%; P = 0.35). In the ACCORD 03 trial, late toxicities were primarily of grade 1 or 2. However, nine patients experienced grade 4 toxicities including necrosis, fistula, bleeding, or pain of whom five were treated with an APR and four underwent colostomy alone.
Chemoradiation in HIV-Positive Patients
HIV-positive patients pose a particular treatment challenge due to their inability to tolerate CMT. Early reports indicated that some patients were receiving less than optimal therapy due to concerns for treatment toxicity. However, patients with a CD4 count of ≥200 can have excellent control of their disease with acceptable morbidity. Those with CD4 counts of <200, however, may require a modification in their treatment regimen such as omission of MMC or a reduction in the RT field and/or dose. UCSF analyzed 17 HIV-positive patients and documented CD4 counts. All nine patients with a CD4 count ≥ 200 had control of their disease. Four patients did require a treatment break of 2 weeks, but no hospitalizations occurred. Among the eight patients with CD4 counts < 200, four experienced lowered blood counts, intractable diarrhea, or moist desquamation. Four of eight ultimately required colostomies for either treatment-related toxicity or for salvage of their disease. Disease, though, was controlled in seven of eight patients. Thus, based on the UCSF experience with HIV-positive patients, one should consider modifying the treatment regimen particularly if CD4 counts are less than 200.
Dose of Radiation
Local-regional failures occur in 20% to 30% after definitive chemoradiation. Because of such local-regional failures, RTOG 92-08, a phase II, dose escalation trial was designed to escalate dose to 59.4 Gy, with a mandatory treatment break of 2 weeks after the initial 36 Gy. The initial study included 47 patients with a mandatory break. The update of RTOG 92-08 analyzed not only the original 47 patients with the mandatory break but also analyzed 20 additional patients who did not have a planned break. Both groups of patients showed no difference in OS or LRF when compared historically to patients on RTOG 87-04 MMC arm. The higher dose likely did not result in improved outcomes because of the treatment break, which may have allowed for tumor repopulation and/or repair of sublethal damage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree