1
Chronic kidney disease is a common complication in diabetes
It is estimated that approximately 40% of individuals with diabetes will develop chronic kidney disease (CKD) during the course of their disease [ , ]. According to the global diabetes prevalence estimates, this translates into 190 million adults living with CKD and type 2 diabetes globally in the year 2021 (IDF atlas https://diabetesatlas.org/en/ ). Thus, the growing numbers of people living with diabetes are largely fueling the CKD epidemics. Moreover, CKD has emerged as one of the leading causes of mortality worldwide. For most noncommunicable causes of death, the increased life expectancy has increased the numbers of death globally, whereas age-standardized death rates have decreased. CKD, however, is one of the few noncommunicable causes of death, where the age-standardized death rates have increased between 1990 and 2013 [ ]. And although the reports showing declining rates of end-stage kidney disease in type 1 diabetes are reassuring [ ], a more detailed analysis shows that the cumulative incidence rates of severe albuminuria actually ceased declining in individuals with type 1 diabetes onset after the 1990s [ ].
2
Clinical manifestation of CKD in diabetes
Diabetes-induced kidney disease typically develops after a diabetes duration of 10 years, or at least 5 years in type 1 diabetes, but may be present already at the time of the diagnosis of type 2 diabetes. The evolution, timing, histological, and clinical presentation of CKD vary considerably in type 1 and type 2 diabetes. This most likely pertains to differences in age and comorbidities, such as hypertension, dyslipidemia, and vascular disease, that usually coexist in type 2 diabetes, whereas in type 1 diabetes, hypertension is merely a consequence of kidney disease [ ]. Histologically, glomerulopathy characterized by thickening of the glomerular basement membrane and mesangial expansion, leading to progressive reduction in the filtration surface of the glomerulus is the most important structural change in type 1 diabetes [ ], whereas nonspecific, tubulo-interstitial and vascular changes are more common in type 2 diabetes [ ].
Clinically, these structural changes are reflected by the occurrence of albuminuria and glomerular filtration rate (GFR) decline. Currently, it is believed that both albuminuria and impaired GFR are complementary, overlapping manifestations of CKD in diabetes [ ], with type 1 diabetes predominantly presenting with a proteinuric phenotype [ ], and as many as 35%–57% of people with kidney disease and type 2 diabetes presenting with a nonproteinuric GFR loss [ ]. Taken together, albuminuria certainly increases the risk of GFR loss, and there are common risk factors for both albuminuria and GFR loss, but these two phenotypes may not always evolve together.
3
Is kidney disease due to diabetes reversible?
Most CKDs progress relentlessly to end-stage kidney disease. Even though we are used to thinking about CKD in diabetes as a progressive noncurable disease, this might not necessarily be the case. A study with pancreas transplantation in individuals with diabetes-induced kidney disease lesions showed that 10 years after the transplantation and long-term restoration of normoglycemia, the glomerular lesions had substantially improved, with most patients’ glomerular structure returning to normal [ ].
In line with that, the seminal Diabetes Control and Complications Trial (DCCT) randomized individuals with newly diagnosed type 1 diabetes to an intensive insulin based or conventional glycemic control arm and followed them for 30 years revealed that those with microalbuminuria (moderate albuminuria) and even macroalbuminuria (severe albuminuria) can revert to normoalbuminuria [ ]. Surprisingly, however, regression from microalbuminuria was not associated with decreased risk of cardiovascular events or estimated GFR (eGFR) reduction, but it may be important to note that most of the regression from microalbuminuria occurred spontaneously even without the use of RAAS inhibition [ ].
4
Who are at risk of CKD?
The key risk factors for CKD development are certainly long-term exposure to hyperglycemia and increasing age. Interestingly, onset of type 1 diabetes between 10 and 14 years of age was associated with a difference in the risk of end-stage kidney disease, depending on the sex, with male sex associated with almost the double risk [ ]. This indicates the role of sex hormones in shaping the CKD risk. Importantly, traditional risk factors, such as increased blood pressure, smoking, obesity, and dyslipidemia, also markedly increase the risk of kidney damage. Intrauterine growth retardation is also associated with an increased risk of CKD, possibly due to a low glomerular filtration area and/or reduced nephron number at birth [ ]. Other recently discovered modifiable risk factors include chronic low-grade inflammation, advanced glycation end-products, and lack of physical activity [ ]. Notwithstanding, huge efforts have been put into exploring the genetic architecture of CKD, yet much of the inherited predisposition to CKD remains unexplained [ ].
5
Screening for CKD in diabetes
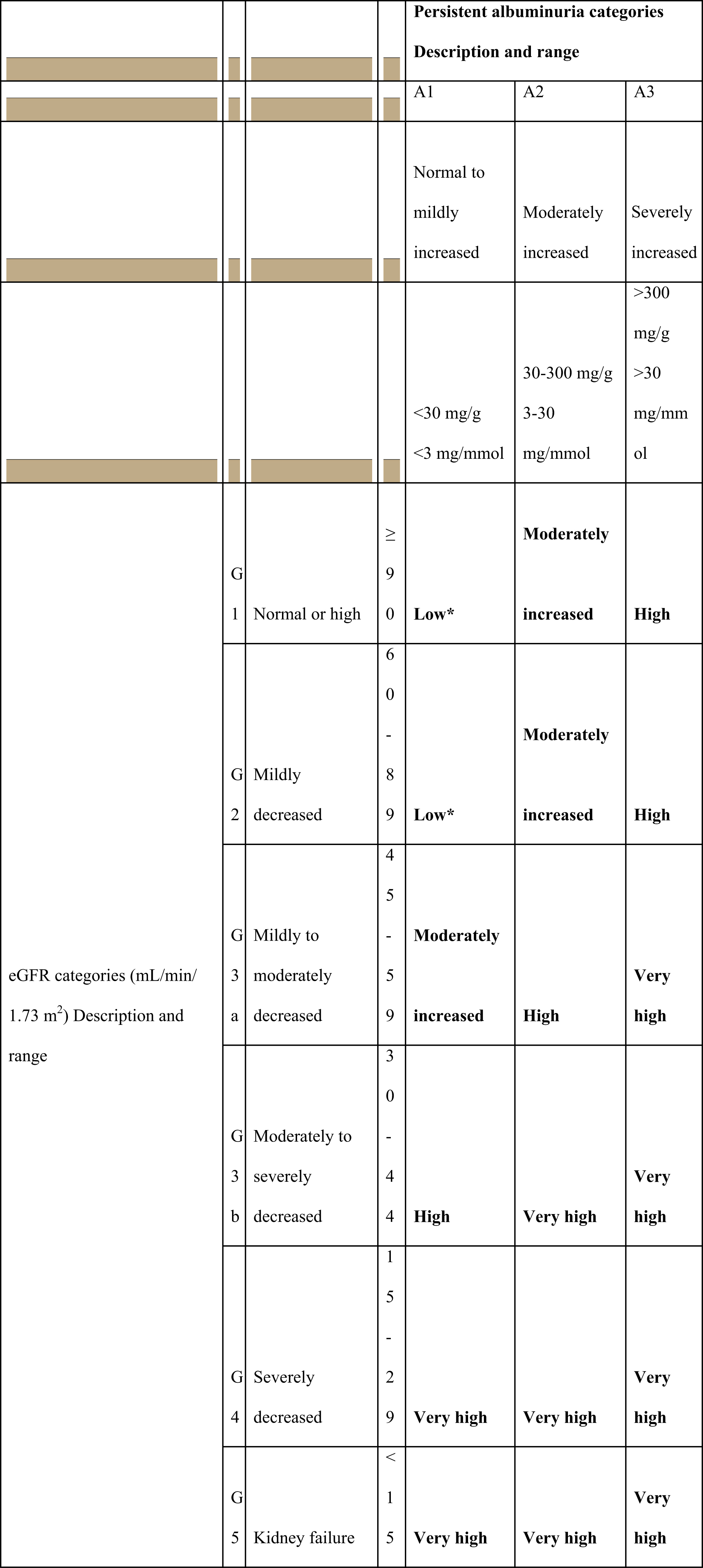
CKD is commonly defined as abnormalities of kidney structure or function, present for more than 3 months, with implications for health [ ]. It is classified based on cause, GFR category, and albuminuria category ( Scheme 17.1 ). In clinical practice, we use two tests for the CKD screening.
5.1
Estimating GFR
GFR is usually estimated by using a serum creatinine-based equation, preferably the CKD-EPI equation. eGFR of persistently less than 60 mL/min/1.73 m 2 is considered as a decreased eGFR [ ]. The settings in which eGFR, estimated by the CKD-EPI equation, may be less accurate, include extremes of body size, pregnancy, with extremes in muscle mass (after limb amputation, in skeletal muscle disease), low/absent meat ingestion, etc. [ ].
5.2
Albuminuria checkup
For the screening of albuminuria, the preferred method is albumin-to-creatinine ratio (ACR) measurement from an early morning urine sample [ ]. Finding is pathological if ACR is ≥30 mg/g (≥3 mg/mmol) [ ]. Pathological ACR should be confirmed on at least two further occasions because of high biological variability of >20% between measurements in urinary albumin excretion [ ]. In addition, strenuous exercise within 24 h, fever, congestive heart failure, marked hyperglycemia, menstruation, and marked hypertension may elevate the ACR independently of kidney damage [ ]. Individuals with two or more positive (≥30 mg/g or ≥3 mg/mmol) ACR tests on early morning samples collected within a 3- to 6-month period should be diagnosed as having persistent albuminuria.
Nevertheless, the criterion of “chronicity” is confirmed if both markers eGFR decrease or persistent albuminuria are present for more than 3 months. Both markers of kidney damage are needed for the screening, since they both independently predict the risk of concurrent CKD complications as well as future cardiovascular outcomes ( Scheme 17.1 ) [ , ]. CKD can be diagnosed also in individuals with normal eGFR and without albuminuria, based on the presence of other markers of kidney damage, such as imaging showing polycystic kidney disease or kidney biopsy abnormalities [ , ].
CKD occurring among people with diabetes is usually attributed to diabetes, unless other causes, coincident with diabetes, are readily evident—like the nephrotic syndrome, abnormal urine sediment, rapid decline of kidney function, short diabetes duration, hematuria, or other nondiabetic kidney disease indicators—and deserve further evaluation [ , ].
Modern diabetes care involves annual screening of all individuals for the presence of urinary albumin concentration and eGFR calculation, starting from the time of diagnosis in type 2 diabetes and 5 years after diagnosis in type 1 diabetes [ ].
6
Screening challenges
Even though screening for CKD is easy, data show that screening is largely suboptimal. In a multicenter study from the US primary care practices, that assessed the CKD prevalence in adult individuals with type 2 diabetes, it was demonstrated that in the 15-month period urine protein and eGFR testing were not performed in 51.4% and 15.2% of individuals, respectively [ ]. Moreover, in cases where urinary and serum creatinine data were available, only 12.1% of persons, fulfilling the diagnostic criteria for CKD, were identified as having CKD by their clinicians. There were no differences in the clinicians’ likelihood of CKD identification based on practice setting, number of years in practice, or self-reported patients seen per week [ ]. Similar real-world data that come from a university hospital setting, showing that laboratory exams were actually performed, but there was no CKD diagnosis made in the medical file [ ]. Failure to identify individuals with CKD is preventing clinicians to implement therapeutic interventions that may prevent kidney disease progression.
7
Why is it important to recognize the presence of CKD?
Several studies have characterized the CKD presence as an important determinant of overall survival in type 1 [ ] as well as in type 2 diabetes. Moreover, persons with a chronically reduced GFR below 60 mL/min/1.73 m 2 are more likely to die of a cardiovascular event than surviving to end-stage kidney disease [ ]. The increase in cardiovascular event occurrence in CKD may be due to common pathophysiologic mechanisms shared by kidney and cardiovascular disease, including inflammation, oxidative stress, insulin resistance, endothelial dysfunction, arterial calcification, activation of the renin-angiotensin-aldosterone system (RAAS), and the biochemical perturbations across the anemia-inflammation-bone mineral axis, among others [ ]. Importantly, individuals with CKD and type 2 diabetes have higher death rates for the same level of GFR compared to individuals without diabetes [ ]. Studies have demonstrated that diabetes with early CKD cuts life expectancy up to 16 years [ ].
8
Special issue: Increased risk of hypoglycemia
While usually not much discussed, the kidney is crucially implicated in different aspects of glucose homeostasis. Therefore, the presence of kidney disease can affect glucose concentrations profoundly and contribute to the increased risk of hypoglycemia, even in the absence of diabetes [ ]. Hypoglycemia is not only a very unpleasant experience that can lead to unconsciousness if left untreated. What is more, a wealth of evidence today strongly argues that severe hypoglycemia is associated with an increased risk of cardiovascular events and overall mortality [ ].
Although the liver is traditionally seen as the main site for gluconeogenesis, kidneys were found to contribute as much as 40% to total body glucose production [ ]. With kidney function decline, the gluconeogenesis, in particular during fasting, is reduced in the kidneys, impairing the main defense mechanism against hypoglycemia ( Table 17.1 ).
| Directly associated with kidney function |
| Decreased gluconeogenesis |
| Decreased insulin clearance in the kidney |
| Uremic toxin-related decreased insulin degradation in peripheral tissues |
| Decreased clearance of exogenous insulin and other glucose-lowering drugs |
| Indirectly associated with kidney function |
| Malnutrition with reduction in glycogen stores |
| Depression |
Together with the liver, the kidney is also the principal site for insulin clearance. After its release from the pancreatic beta-cells, the insulin concentrations in the portal circulation are fourfold higher than in the systemic circulation [ ]. The liver then removes about 50% already during the first portal passage, whereas the kidney clears most of the insulin in the systemic circulation, mainly by glomerular filtration and proximal tubular reabsorption and degradation [ ]. In healthy individuals, it is estimated that 6–8 units of insulin are removed and degraded by the kidney every day. The role of the kidney in the insulin degradation is even more important after exogenous insulin application as it occurs in diabetes since the insulin concentration is disproportionally higher in the systemic circulation than in the portal vein.
As kidney function declines, to maintain the kidney insulin clearance, the peritubular insulin uptake increases first. However, when GFR falls below 15–20 mL/min, the peritubular uptake cannot compensate for the reduced insulin filtration and reabsorption anymore, and consequently, the metabolic clearance rate of insulin falls precipitously [ ]. Besides, uremic toxins, that start to accumulate during kidney function decline, may also cause inhibition of the insulin degradation in nonkidney tissues, especially in the liver, thus prolonging insulin half-life [ ].
The decline in the kidney function in CKD does not only play a key role for the insulin clearance but also for the clearance of most other drugs used for the treatment of hyperglycemia, therefore making glucose control in these individuals rather challenging. In the setting of increased hypoglycemia risk in individuals with impaired kidney function, choosing medications that do not increase the risk of hypoglycemia, like metformin, sodium glucose transporter-2 (SGLT-2) inhibitors, glucagon-like peptide −1 (GLP-1) receptor agonists, thiazolidinediones, and dipeptidyl peptidase-4 (DPP-4) inhibitors, is of particular importance [ ].
9
“The 5-finger rule” for the prevention and treatment of kidney disease
The care of individuals with diabetes and CKD is multifaceted and complex. The core of CKD prevention and management can be figuratively summarized by a “5-finger rule,” as outlined in Table 17.2 . The same can be reiterated by bringing forward four pillars of care that are based on healthy lifestyle and diabetes education, as outlined in new American Diabetes Association guidelines when speaking of reduction in risk of diabetes complications [ ]. The rationale for kidney disease prevention and treatment is similar and will be covered together, with any important distinction between both specifically highlighted.
| Finger | Risk factors | Target level for most individuals | Treatment – special considerations |
|---|---|---|---|
| 1 | Hyperglycemia | HbA1c <7% [ ] | Use agents with low risk of hypoglycemia; agents and doses need to be adjusted to eGFR |
| 2 | Increased blood pressure | Below 130/80 mmHg [ ] | RAAS inhibition is the standard care |
| 3 | Dyslipidaemia | LDL-cholesterol below 1.4 mmol/L (very high cardiovascular risk) or below 1.8 mmol/L (high cardiovascular risk) and at least 50% reduction from the baseline [ ] | Use statin or statin/ezetimibe combination; do not initiate statin therapy in dialysis-dependent CKD |
| 4 | Lifestyle: Healthy diet, physical activity, weight management, smoking cessation |
| Increased exercise intensity may be of additional benefit for CKD prevention |
| 5 | Organ protection | Use therapies with proven effects for kidney protection [ ] | RAS inhibitors, iSGLT-2, nonsteroidal MRA (finerenone) |
9.1
First finger: Optimal glycemic control
The fundamental abnormality in diabetes is abnormal glucose metabolism. Hence, optimal glycemic control is crucial for the prevention of kidney damage and deterioration in GFR [ , ]. Importantly, seminal studies in both type 1 [ ] and type 2 diabetes [ , ] have demonstrated a durable positive effect of intensive glucose control on kidney outcomes. In particular, intensive glycemic control early in diabetes translates into long-term kidney benefits [ , ], described in the medical literature as “legacy effect” or “metabolic memory.” This phenomenon was also confirmed in real-world studies [ ]. The effect is less clear in individuals with established diabetes [ ], with long-term diabetes dampening the “legacy effect.” The mechanism behind this effect is not completely understood. It is hypothesized that it involves epigenetic modifications of the endothelium through histone modification, DNA methylation and noncoding RNAs [ ]. These modifications to the endothelium are then driving the changes in the microvasculature, leading to microvascular disease. In addition, hyperglycemia induces mitochondrial superoxide anion overproduction, leading to downstream increase in the production of advanced glycation end-products, triggering and perpetuating inflammatory pathways.
However, with advanced kidney disease, the optimal glycemic control level is less clear [ ], with most guidelines recommending a target glycated hemoglobin level of approximately 7% but not below 7% [ ].
Today, accumulating research findings highlight that even transient glucose spikes may suffice to elicit continuous changes in the metabolic milieu perpetuating target organ damage [ ]. Even transient hyperglycemia can induce accumulation of senescent cells [ ]. Therefore, it is not only the average blood glucose mirrored by glycated hemoglobin that affects kidney outcomes but also other parameters of glucose exposure, in particular glucose variability, that may be important in the assessment of the role of glycemic control for target organ damage [ ]. In this context, the KDIGO guidelines from 2020 emphasized the need for regular self-blood glucose monitoring or the use of continuous glucose monitoring systems in cases where treatment, associated with higher risk of hypoglycemia, is used, for example, with sulfonylureas and insulin [ ]. Namely, in the context of advanced CKD, the use of these medications increases the risk of hypoglycemia. Regular monitoring of glucose in this setting improves the safety of antihyperglycemic therapy by providing data on glucose fluctuations that can help in avoiding hypoglycemia.
Moreover, the choice of glucose-lowering agent should not be accidental in CKD according to the new KDIGO guidelines. The first-line therapy consists of metformin and SGLT-2 inhibitor (iSGLT-2) [ ]. If additional therapy is needed, agonists of the glucagon-like peptide-1 receptor (GLP-1 RA) are recommended [ ]. Recommendations are based on glycemic efficacy, safety, and additional kidney and cardiovascular beneficial effects [ ]. Later, DPP-4 inhibitors, thiazolidinediones, sulfonylurea, or insulin may be added, if needed.
9.2
Second finger: Optimal blood pressure control
Elevated blood pressure is a strong risk factor for the development and progression of CKD in both type 1 and type 2 diabetes, and the benefit of RAAS blockade for slowing kidney disease progression with or without diabetes is well established [ ]. Although the use of all classes of antihypertensive agents is recommended for blood pressure lowering to be achieved, RAAS inhibition with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) remains a cornerstone of standard-of-care in diabetes, mainly due to its additional kidney protective effects [ ]. In hypertensive people with type 1 and type 2 diabetes, ACE inhibitors and ARBs reduce albuminuria, the risk of progression to end-stage kidney disease, and the risk of cardiovascular events [ , , ]. However, two long-term randomized controlled trials have demonstrated no kidney protective effect of either ACEi or ARBs in normotensive individuals with normo- or microalbuminuria in type 1 or type 2 diabetes [ ].
It is widely accepted, that in hypertensive individuals, the blood pressure lowering is of central importance for slowing GFR loss [ ]. The current blood pressure target is set at below 130/80 mmHg for the majority of individuals with diabetes in most of the international clinical practice guidelines [ , ]. In the setting of severe albuminuria (≥300 mg/g creatinine), even lower blood pressure targets may be suitable, provided that changes in eGFR are monitored regularly [ ].
It is of note that dual RAAS blockade with ACEi and ARBs was associated with reduction of albuminuria in randomized controlled trials in individuals with diabetes but also with increased rates of hypotension, hyperkalemia, and acute kidney injury. Therefore, current data speak against its use in individuals with diabetes [ , ].
9.3
Third finger: Optimal lipid control
Data support an active role of lipids for kidney disease development. In particular, elevated triglycerides, non-high-density lipoprotein cholesterol, apolipoprotein-B-100 or low high-density lipoprotein (HDL) cholesterol concentrations are independently associated with the development of CKD [ ]. However, conventional lipid measurements do not fully account for the complex lipid changes associated with CKD, like triglyceride enrichment across the lipoprotein particles, increased sphingomyelin levels, and imbalance of different apoprotein levels [ ]. Statin treatment with or without ezetimibe is the mainstay approach for complex lipid abnormalities present in CKD [ ]. Statin treatment was also one of the pillars of the integrated multifactorial treatment in individuals with type 2 diabetes and microalbuminuria from the STENO-2 study, demonstrating the importance of early aggressive multifactorial treatment in reducing microvascular as well as macrovascular diabetes complications [ ]. Newer lipid-lowering therapies with proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors or the small interfering RNA, directed against PCSK9, have not been used in clinical trials, that were designed particularly for individuals with CKD. However, those individuals with CKD, included into randomized controlled trials targeting PCSK9, have shown similar cardiovascular benefits in those with CKD compared to those without CKD, with comparable safety [ , ]. Nevertheless, proper dietary advice and support in dietary alterations may be helpful in optimizing lipid control [ ]. Newer therapies and approaches, however, are needed to fully address the complex lipid abnormalities in CKD and to prevent their deleterious effects.
9.4
Fourth finger: Optimal lifestyle
Lifestyle factors, such as physical activity, healthy diet, and smoking, affect CKD outcomes profoundly. Regular moderate-to-vigorous physical activity was associated with reduced incidence and progression of CKD in type 1 diabetes, as well as reduced risk of cardiovascular events and mortality [ , ]. Therefore, regular moderate-to-vigorous exercise should become a central part of the management of individuals with type 1 diabetes, in the absence of contraindications and accompanied with all needed educational support for optimal concomitant hyperglycemia management. Similarly, results point in the same direction in type 2 diabetes [ ].
Also, the need for embracing a healthy diet, high in vegetables, fruits, whole grains, fiber, legumes, plant-based proteins, unsaturated fats, and nuts and lower in processed meats, refined carbohydrates, and sweetened beverages, is emphasized, although the number of high-quality studies, analyzing the effects of different diets among individuals with diabetes and CKD, is small [ ]. In particular, salt intake should be <5 g per day and protein intake maintained at 0.8 g of protein/kg body weight per day for those with diabetes and non-dialysis-dependent CKD; however, it should be maintained at 1.0–1.2 g of protein/kg body weight per day for those treated with peritoneal or hemodialysis, to prevent increased catabolism and malnutrition [ ].
Obesity adversely affects the major risk factors associated with CKD, including lipid, blood pressure, and glucose control, as well as promoting insulin resistance. Obesity also has direct effects on the kidney, including changes in intraglomerular hemodynamics, increased sympathetic activity, inflammation, and altered expression of growth factors. Although epidemiologic data on the association of BMI with kidney disease in diabetes gave inconclusive results, with the Mendelian randomization approach genetic evidence was provided for a causal link between obesity and DKD in type 1 diabetes [ ]. This finding has widespread and profound consequences as the obesity prevalence rises, pointing toward a dramatic increase in CKD prevalence unless interventions occur. With the availability of effective weight-losing therapies, like GLP-1 receptor agonists (e.g., semaglutide) or combined GLP-1/GIP (glucose-dependent insulinotropic polypeptide) agonist tirzepatide, effective weight management is not so elusive anymore and becomes an important treatment target. Treatment with these agents has already shown beneficial effects by lowering albuminuria [ , ], yet dedicated studies on their long-term effects on kidney function in individuals with CKD are still lacking.
Several studies, on the other hand, have highlighted that smoking is a strong risk factor for kidney disease progression and also, that the risk increases with the increasing dose of smoking [ ]. In addition, smoking is strongly associated with increased risk of cardiovascular disease. Therefore, smoking cessation should be encouraged by every encounter with those who smoke.
9.5
Fifth finger: Organ protection
Despite optimized glycemic and blood pressure control in individuals with CKD, there is still an unmet need to reduce the risk of progression to end-stage kidney disease and the development of related cardiovascular events. Fortunately, as the understanding of the CKD in diabetes broadens, new targets and treatment possibilities develop.
Substantial evidence has accumulated that iSGLT-2 confer significant kidney and cardioprotective effects in individuals with type 2 diabetes and CKD. They reduce intraglomerular pressure, albuminuria, and slow GFR loss through mechanisms, independent of glycemia [ ]. Also, they reduce oxidative stress in the kidneys by >50%, blunt increase in angiotensinogen level, decrease NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome activity, and improve renal oxygenation [ , ]. Some large clinical trials have confirmed the beneficial effects of iSGLT-2 treatment in individuals with CKD [ ], some even without diabetes [ , ], on composite kidney outcomes or cardiovascular mortality. Therefore, iSGLT-2 should be used preferentially as the glucose-lowering medication of choice, in individuals with diabetes and CKD, to reduce the risk of CKD progression and cardiovascular events, provided that the eGFR is above 20 mL/min at their initiation [ ]. Moreover, some studies also suggest a possible protective role for a composite adverse kidney outcome by iSGLT-2 dapagliflozin in the early prevention of kidney disease, like in individuals with normoalbuminuria [ ]. Recently, in the EMPA-KIDNEY trial in individuals with and without diabetes at different stages of albuminuria, empagliflozin had a significant effect on the annual change in eGFR decline compared with placebo [ ].
In addition, some GLP-1 RA or combined GIP/GLP-1 RA tirzepatide have a direct effect on the kidney and have also been reported to improve kidney outcomes, largely driven by a decrease in albuminuria, compared with placebo [ , ]. However, we still do not have the results of a trial with GLP-1 RA in a population, selected for CKD or in which kidney disease is the primary outcome. Nevertheless, many GLP-1 RA have proved their cardioprotective effects and should be used for cardiovascular risk reduction in individuals at high cardiovascular risk [ , ].
Another agent, from the group of nonsteroidal mineralocorticosteroid receptor antagonists (MRA), finerenone, has arisen as a novel disease modifying drug for individuals with type 2 diabetes and CKD. In two large clinical trials, FIDELIO-DKD and FIGARO-DKD, finerenone was shown to slow down the progression of CKD and also decrease the risk of cardiovascular events and mortality [ , ]. The risk of hyperkalemia with the nonsteroidal MRA finerenone was significantly lower compared to the use of older steroidal MRAs.
10
Conclusion
Although CKD is common in diabetes and its consequences are grim, large long-term clinical trials have demonstrated that strategies implementing improved blood glucose and blood pressure control retard the development and progression of kidney disease. Moreover, in recent years, the armamentarium of drugs, directly improving kidney function, have increased substantially. With this, systematic and regular screening for CKD has really become a “sine qua non” , since failure to detect CKD may translate into failure to treat CKD properly ( Table 17.3 ). Indeed, because of treatment improvements the natural history of CKD in diabetes has changed over the last decades prolonging time to end-stage kidney disease and decreasing cardiovascular disease burden and mortality at the same time.