Original equation:
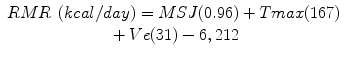
Mifflin-St Jeor [6]
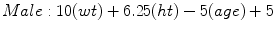
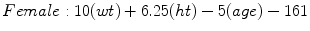
(RMR = resting metabolic rate, Tmax = maximum body temperature, Ve = minute ventilation, wt = weight in kilograms, ht = height in meters) [6]
The American College of Chest Physicians (ACCP) consensus statement in 1997 recommends 25 kcal/kg of usual body weight for calculating resting energy expenditure to avoid overfeeding. The calculation for obese individuals (BMI >25 using ACCP definitions) should utilize ideal body weight (Male IBW = 50 kg + 2.3 kg for each inch >60 in., Female IBW = 45.5 kg + 2.3 kg for each inch >60 in.). Underweight patients (BMI <16) are at increased risk for refeeding syndrome. The calculation for REE in this setting should utilize the actual body weight for the first 7–10 days and then the ideal weight thereafter. For example, a 70 kg male with a BMI of 25 has a resting energy expenditure of 70 kg × 25 kcal/kg or 1,750 kcal [8].
Metabolic Requirements
Physiologic Changes
While aging decreases the basal metabolic rate (BMR), physiologic aging does not progress at the same rate in all individuals. It is agreed that between the ages of 30 and 70 years, the BMR decreases by approximately 16 % and body composition changes such that there is increased fat and decreased protein content [9, 10]. Lean body mass for the average 30-year-old is approximately 45 % of total body weight (TBW) and for a 70-year-old is approximately 27 % TBW, while the total body fat is 14 % of TBW for age 30 and 30 % of TBW for age 70 [11].
A number of factors impact on appetite as patients’ age. The incidence of dysgeusia and dysosmia increases secondary to the decreasing number of taste buds, peripheral olfactory atrophy, and less saliva production. The first taste buds to be lost detect sweet and salt quality with bitter and sour diminishing later. Due to these changes food begins to taste bitter and not smell appetizing [3, 9, 12]. Additionally, the physiologic regulation of appetite is a complex neural and hormonal network involving the autonomic nervous system, enteric nervous system, and hypothalamic-pituitary-adrenal axis in homeostatic balance. This balance is disrupted with chronic diseases, cancers, inflammatory processes, and polypharmacy, resulting in an overall decrease in appetite [12].
Renal function declines with age and 40 % of nephrons are sclerotic by the age of 85. This process is accelerated in patients with diabetes, hypertension, dyslipidemia, and/or atherosclerosis. As renal function declines, the ability to regulate fluid balance and acid/base status deteriorates, and dehydration can occur as the kidney is unable to respond to renal sodium and water losses. This is likely secondary to a decreased responsiveness to ADH and decreased renin-angiotensin system activity, as well as diminished thirst [3, 13].
The gastrointestinal tract also undergoes age-related changes that include esophageal dysmotility from a change in peristalsis, chronic atrophic gastritis, intestinal bacterial overgrowth, and chronic constipation. All of the above-mentioned factors, as well as laxative abuse, impact the intake and absorption of nutrients [9, 10]. Dyspepsia frequently occurs in the elderly and is most commonly related to peptic ulcer disease, gastroesophageal reflux, or gastric cancer [13]. Lactase, an essential enzyme in the digestion of dairy products, is produced in lesser amounts in the elderly, which leads to lactose intolerance resulting in stomach cramps and diarrhea. These factors contribute to malnutrition and vitamin/mineral deficiencies in the geriatric population [3]. Malnutrition, in turn, increases the incidence of complications such as wound failure/infection and nosocomial infections when combined with age-related reductions in skin integrity and immunocompetence. In an ICU setting these complications are associated with negative outcomes and potentially death [9, 10].
Common Deficiencies
The aging population is at risk for deficiencies in many vitamins and nutrients. Decreased appetite, the inability to chew certain foods (e.g., fresh fruits and vegetables), and the increased incidence of lactose intolerance are contributing factors to these deficiencies (Table 33.1).
Deficiency | Etiology | Symptoms/signs | Recommendation |
|---|---|---|---|
Vitamin D | 1. Increased incidence of lactose intolerance | Bone pain, muscle weakness | 600 IU |
2. Decreased intake | |||
3. Decreased sunlight exposure | |||
4. Decreased renal conversion of vitamin D to the active form | |||
Vitamin B12 | 1. Decreased intake of red meat due to cost, difficulty with mastication, caloric restriction | Anemia, neuropathy, dementia | Adequate dietary intake with supplementation if necessary |
2. Atrophic gastritis | |||
Vitamin K | 1. Anticoagulant use | Easy bruising/bleeding, hematuria, hematochezia | Adequate dietary intake with supplementation if necessary |
2. Antibiotic use | |||
3. Sulfa medications | |||
Calcium | 1. Hormonal changes in women (postmenopausal) | Osteoporosis | 800–1,200 mg/day |
2. Inadequate intake | |||
Water | 1. Diminished thirst sensitivity | Altered mental status, hypotension | 30 cc/kg/day |
2. Fluid loss through diarrhea/vomiting not replaced |
Vitamin D deficiency in the elderly is multifactorial with decreased consumption of vitamin D-fortified dairy products occurring secondary to an increased incidence of lactose intolerance. Further, less exposure to sunlight disrupts the conversion of vitamin D to the active form. This can be more pronounced in institutionalized patients who have limited exposure to direct sunlight. Finally, as the kidney ages, its ability to convert vitamin D to the active form also decreases. In order to prevent vitamin D deficiency, it should be supplemented (600 IU daily) in the diet, and efforts should be directed at providing the elderly with more exposure to sunlight [11, 13].
Red meat is the primary source of vitamin B12, and deficiencies may be related to food cost, dietary caloric restrictions, and difficulty with mastication due to poor dentition. Twenty percent of the geriatric population are deficient in vitamin B12 [13]. Decreased gastric acid secretion related to aging and/or the use of acid-reducing medications diminishes intrinsic factor production which can lead to atrophy of the gastric mucosa. Intrinsic factor is essential for the release of vitamin B12 from its carrier protein as well as absorption. Signs and symptoms of vitamin B12 deficiency include anemia, neuropathy, and dementia [11, 13].
Vitamin K deficiency is not prevalent in the geriatric population; however, certain medications may lead to an inadequate absorption such as anticoagulants, antibiotics, and sulfa drugs. This vitamin should be kept in mind when starting these medications in the intensive care setting [11].
Nutritional Monitoring
There is no standard way to measure malnutrition in the hospitalized elderly patient. There are screening tools to help identify those individuals at risk. Screening is important due to the adverse effects of malnutrition on outcomes. Patients presenting malnourished have increased hospital stays, more complications, and higher mortality rates. In this section, we will discuss risk factors, screening tools, and biochemical markers of malnutrition.
Risk Factors
Risk factors for malnutrition in the elderly include those unrelated to age and those related to age [2]. Factors unrelated to age include cancer, chronic and severe organ failure, gastrointestinal diseases, alcoholism, chronic infectious and/or inflammatory diseases, as well as all factors likely to cause one or more of the following: a reduction in food intake, an increase in energy requirements, and malabsorption.
Risk factors related to age include psychological, social and environmental factors such as depression, grieving, financial hardship, and admission to a long-term care facility. Oral, dental, and swallowing disorders can contribute to malnutrition. Dementia and other neurological disorders put the elderly at increased risk. Acute problems that result from trauma such as pain, fractures, and surgery increase the risk for malnutrition the elderly.
Screening Tools
Screening for malnutrition includes evaluating risk factors, appetite and food intake, comparing weights as possible, and calculating body mass index. These may contribute to the diagnosis of malnutrition, but will not be obtainable in the obtunded trauma patient.
The Mini Nutritional Assessment (MNA) has been used as a standard for screening the elderly for malnutrition risk and is recommended in the 2007 evidence-based guideline from France. Questions address various areas and include appetite, meals, and weight loss. It also contains objective measurements of calf and midarm circumference. There is also a short form, the MNA-SF, which contains only 6 items [38]. An even shorter subset focusing on dietary habits, called the MNA-3, is suggested as the most important component of screening [14].
Albumin
Visceral protein stores can be assessed by serum albumin concentration. Albumin is a plasma protein that maintains plasma oncotic pressure and a carrier protein for multiple elements and drugs. Testing serum levels is routinely available and the normal range is between 3.5 and 5 g/dL. The half-life of serum albumin is approximately 20–21 days; therefore, loss of protein stores will be reflected in the serum level at that time. Serum albumin is a reliable marker in the absence of liver disease, renal disease, prolonged bed rest, infection/sepsis, and cancer. However, these ailments are frequently comorbidities for many of the geriatric patients that enter critical care units [11, 15].
The assay of C-reactive protein may assist in interpreting the albumin result when an inflammatory process is involved. Serum albumin may distinguish between two forms of malnutrition: that due to a deficiency in food intake (albumin may be normal) and that due to inflammation and a catabolic state, with a rapid fall in serum albumin [2].
Transferrin
Transferrin is a protein that binds iron for transport to the bone. Its levels are affected by total body iron storage [15]. With aging, body tissue iron stores increase leading to a decrease in transferrin levels in healthy individuals. However, malnourished elderly patients with decreased protein and low iron stores may express a normal transferrin level. The 8–10-day half-life makes transferrin a good choice to assess the nutritional status of younger individuals, but should not be used in the geriatric population [10]. Normal serum values are between 200 and 400 mg/dL [15].
Prealbumin
Prealbumin is a visceral protein synthesized by the liver that binds thyroxine. The half-life of prealbumin is approximately two days making it a promising biochemical marker of protein storage and ideal for short-term nutritional assessment. Normal serum levels are between 18 and 40 mg/dL [11, 15].
Total Lymphocyte Count
Depressed total lymphocyte count can be a marker of malnutrition as it is used as a measure of immunocompetence. However, the total lymphocyte count can be altered by a wide variety of factors including hypoalbuminemia, infection, chronic comorbid conditions, and malignancy. The normal range is greater than 1,500 cells/mm3 [11]. Total lymphocyte count is not an adequate indicator of nutritional status in the geriatric populations as it is affected by the factors mentioned above [16].
Diagnosis of Malnutrition
Per the French Guideline on Protein-Energy Malnutrition, the diagnosis of malnutrition is based on one or more of the following criteria: [2] weight loss ≥5% in 1 month or ≥10% in 6 months, body mass index <21 (a BMI ≥21 does not exclude the diagnosis of malnutrition), serum albumin concentrations <35 g/L, and MNA score <17.
Severe malnutrition may be diagnosed by one or more of the following criteria:
weight loss of ≥10% in 1 month or ≥15% in 6 months, BMI <18 and serum albumin <30 g/L. Severe malnutrition requires rapid nutritional management.
Nutritional Requirements (See Table 33.2)
Variable | Requirement | 70 kg nonstressed patient | 70 kg ICU patient |
|---|---|---|---|
Calories | 25 kcal/kg/day | 1,750 kcal | 1,750 kcal |
Protein | 1.0–1.5 g/kg/day | 70 g = 280 kcal | 105 g = 420 kcal |
Fat | 20–30 % daily caloric intake (20 %) | 38 g = 350 kcal | 38 g = 350 kcal |
Carbohydrates | Makes up the rest of daily caloric intake | 1,750 – (280 + 350) = 1,120 kcal [kcal/4 = 280 g] | 1,750 – (420 + 350) = 980 kcal [kcal/4 = 245 g] |
Intravenous fluid | 25–30 cc/kg/day | 2,100 cc/day | 2,100 cc/day |
Vitamin E | 10 IU/day | ||
Vitamin C | 200 mg/day | ||
Zinc | 2.5–5 mg/day | ||
Copper | 0.3–0.5 mg/day | ||
Selenium | 20–60 mcg/day |
Carbohydrates
Half of the average Western diet consists of calories derived from carbohydrates. The body is able to store approximately 1,200 cal in the liver and muscle in the form of glycogen. These stores are immediately available and depleted within three days of starvation. This process begins between 8 and 16 h postprandially. Glycogenolysis occurs as insulin levels decrease, mobilizing glucose from hepatic stores. Alanine is also essential for this process in muscle and is used for gluconeogenesis as muscle cannot mobilize glucose from glycogen for lack of glucose-6-phosphatase [12]. The ability to metabolize glucose diminishes with age, leading to chronically elevated blood glucose levels and the development of advanced glycosylation end products (AGEs). AGEs promote fibrosis, decrease connective tissue flexibility, and change the extracellular matrices of the heart, kidney, skin, and central nervous system. These changes result in many of the common comorbid conditions in the geriatric population: neuropathy, nephropathy, cardiomyopathy, atherosclerosis, etc [12]. Therefore, it is recommended that elderly individuals consume complex carbohydrates rather than simple sugars [11].
Proteins
The average adult requires 0.8 g of protein per kilogram body weight daily. The body is able to store protein in large amounts. However, only 50 % of the proteins can be utilized without serious consequences. Over 50 % depletion of protein stores is incompatible with life [12]. In the stress state, the body may require up to 1.5 g/kg/day to support wound healing, immune function, etc. Furthermore, patients whom are bedbound, or institutionalized, require more than average protein to maintain nitrogen balance. This is not intuitive as one may believe that since lean muscle mass decreases in the elderly population, protein requirements would follow suit. However, the amount of nitrogen retained by the body decreases with decreased caloric intake, and in order to maintain positive nitrogen balance, additional protein must be provided. The average elderly person requires 1.0 g/kg/day of protein in order to maintain skeletal muscle protein metabolism which requires a larger amount of essential amino acids [11, 17]. Finally, only 25 % of undernourished patients achieve protein and energy requirements by day 4 of their hospitalization [18]. Protein intake is essential and should not be overlooked.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree