Gram-positive aerobic bacteria
Bacillus sp.
Coagulase-negative staphylococci
Enterococci
Group A, β-hemolytic streptococcus
Group B streptococcus
Staphylococcus aureus
Gram-negative aerobic bacteria
Acinetobacter spp.
Citrobacter spp.
Enterobacter spp.
Escherichia coli
Haemophilus influenza
Klebsiella spp.
Pasteurella multocida
Proteus spp.
Pseudomonas aeruginosa
Serratia spp.
Anaerobic bacteria
Bacteroides spp.
Clostridium spp.
Fusobacterium spp.
Peptostreptococcus spp.
Prevotella spp.
Marine Vibrio spp.
Vibrio alginolyticus
Vibrio damsela
Vibrio parahaemolyticus
Vibrio vulnificus
Atypical
Mycobacterium kansasii
M. chelonae
M. smegmatis
Fungi
Aspergillus spp.
Candida spp.
Rhizopus
Location
NSTI can affect any part of the body. For simplicity, the body regions most commonly affected are usually categorized in order of decreasing incidence: extremities, perineum and buttocks, trunk, and head and neck. The body region affected varies according to the study patient population, for example, IV drug abusers are disproportionately affected in the extremities at the sites of injection [15–17, 20], while Fournier’s gangrene is seen most commonly in diabetics [20, 33]. Fournier’s gangrene, named after French dermatologist Jean Alfred Fournier described a series of five male patients in 1883, is the eponymous name used to describe NSTI of the perineum (Figs. 17.1 and 17.2). This form of NSTI affects men ten times more commonly than women. NSTI of the head and neck is most commonly preceded by a dental infection [46, 47].
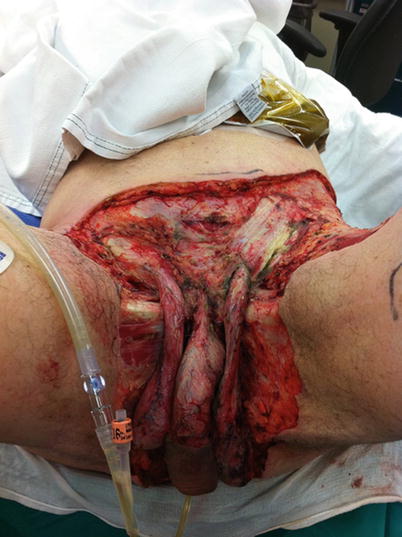
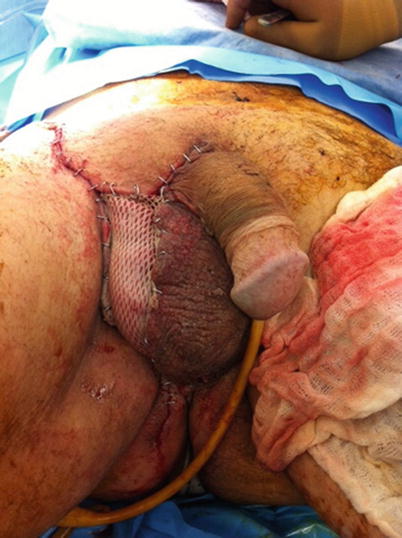

Fig. 17.1
Fournier’s gangrene after radical debridement. Author’s collection

Fig. 17.2
Fournier’s gangrene after partial wound closure and skin grafting. Author’s collection
NSTI can occur following insect bites and muscle strains. Non-recreational drug use needle injection-related NSTI has been reported after tattooing [18], acupuncture [30, 48], and insulin administration [30] (Figs. 17.3 and 17.4). Case reports of postsurgical NSTI have been reported for appendectomy, inguinal hernia, various gynecologic operations, orthopedic fixation, dental procedures, transanal procedures, urologic procedures (including circumcision), angiographic interventions, and laparoscopic procedures [6, 49, 50]. As a general rule, infections adjacent to mucous membranes (oral cavity, rectum, vagina) are caused by the normal resident flora of those mucous membranes, while infections in distant areas are usually caused by resident skin flora [51].
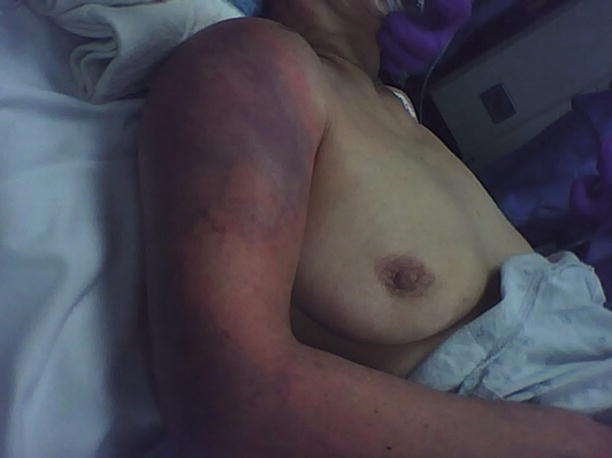
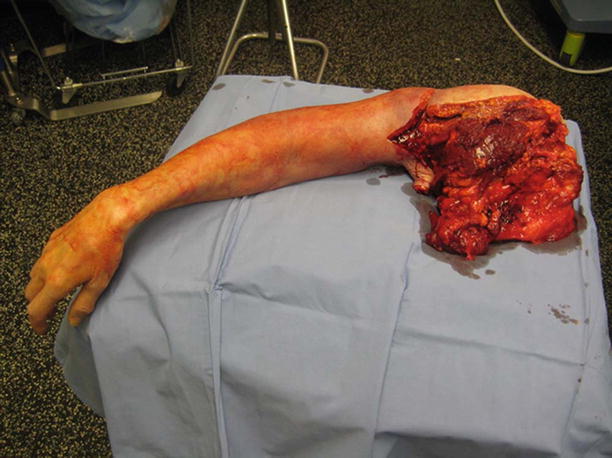

Fig. 17.3
Preoperative appearance of upper extremity NSTI. Author’s collection

Fig. 17.4
Life-saving upper extremity amputation required for NSTI. Author’s collection
Diagnosis
The distinction between NSTI and non-necrotizing infections is of prime importance, the latter being treated with antibiotics alone. The main differential diagnosis includes simple cellulitis, erysipelas, abscess, Clostridial and non-Clostridial myonecrosis, toxic epidermal necrolysis (TEN), staphylococcal scalded skin syndrome, and rarely, cutaneous anthrax (Table 17.2).
Table 17.2
Differential diagnosis of NSTI
Abscess |
Anthrax (cutaneous) |
Cellulitis |
Erysipelas |
Lymphedema |
Myonecrosis (Clostridial and non-Clostridial) |
Myxedema |
Noninfectious fasciitis |
Phlegmasia cerulea dolens |
Staphylococcal scalded skin syndrome |
Toxic epidermal necrolysis (TEN) |
Clinical Exam
The physical exam findings in NSTI have been known since the first description by Hippocrates: soft tissue edema (71–83.7 %) [11, 17, 25], erythema (52–85.6 %) [11, 13, 17, 25], and skin blebs and bullae (13.3–44.9 %) [11, 13, 15, 17, 23, 25]. Crepitus and skin necrosis, highly specific signs, are present in less than a third of patients [5, 33]. The symptom most characteristic of NSTI is severe pain (often out of proportion to exam) (54.7–86.6 %) [11, 16, 17, 23, 25]. Absence of pain and numbness may result, however, from destruction of cutaneous nerves. Systemic symptoms such as fever (32.5–60 %) and hypotension are variably present [11, 13, 17, 23, 25, 30]. No single physical finding is universally present. Reliance upon the physical exam for diagnosis may result in delayed recognition and treatment [12, 43]. In fact, NSTI is the admitting diagnosis in only a minority of confirmed cases [8, 23].
Radiology
Plain Films
Plain films were once considered essential in the diagnostic work-up of NSTI. In recent series, however, soft tissue gas evident on X-rays is present in only about 30 % of cases of confirmed NSTI [11, 15, 30]. This is likely due to the presence of non-gas forming pathogenic bacteria and variable stages of disease upon presentation. While the presence of subcutaneous emphysema is very specific and can confirm the diagnosis when clinically suspected, the absence of subcutaneous gas is not sufficiently sensitive to rule out the diagnosis of NSTI (Figs. 17.5 and 17.6). Awaiting the results of plain films should not delay surgical consultation or intervention.
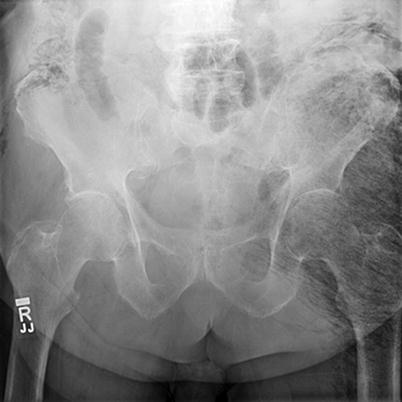
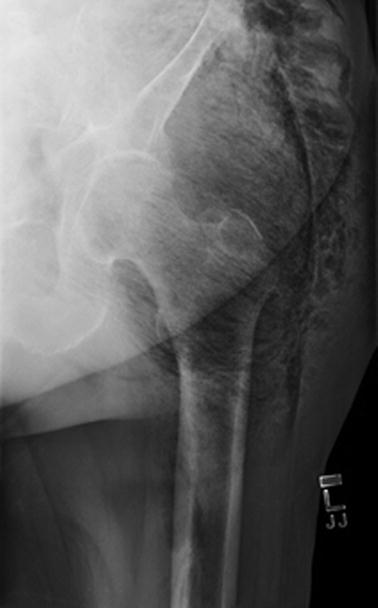

Fig. 17.5
Subcutaneous emphysema evident on plain films. Author’s collection

Fig. 17.6
Subcutaneous emphysema evident on plain films. Author’s collection
Ultrasound
The use of ultrasonography (US) in the diagnosis of NSTI has been described in several single institution studies. Findings characteristic of NSTI include “a diffuse thickening of the subcutaneous tissue, accompanied by a layer of fluid accumulation more than 4 mm in depth along the deep fascial layer when compared with the contralateral position on the corresponding normal limb” [52]. Preliminary work in cadavers suggests that ultrasound is accurate in the detection of subcutaneous air [53].
Using operative and histological findings as the reference standard, Yen et al. report a sensitivity of 88.2 %, specificity of 93.3 %, positive predictive value (PPV) of 83.3 %, negative predictive value of 95.4 %, and an accuracy of 91.9 % [52]. Limitations of this modality include the dependence on operative experience and availability. Until larger, multicenter studies confirm these preliminary results, routine use of US for the diagnosis of NSTI cannot be recommended.
Computed Tomography (CT)
The literature on the use of CT to aid in the diagnosis of NSTI is conflicting; earlier studies reporting inaccuracy are limited by older CT technology and small sample sizes. Newer generation scanners (16-slice and above) have increased sensitivity in detecting pathological changes associated with NSTI: asymmetrical and diffuse areas of soft tissue inflammation and ischemia, muscle necrosis, gas across tissue planes, and fluid collections (Fig. 17.7). Using these four CT criteria, Zacharias et al. report a sensitivity of 100 %, specificity 81 %, PPV 76 %, and NPV 100 % [14]. Thus, the utility of CT seems to be greatest in ruling out NSTI in clinically equivocal cases, thus avoiding unnecessary operative explorations (Figs. 17.8, 17.9, and 17.10).
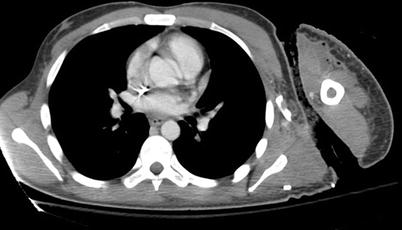
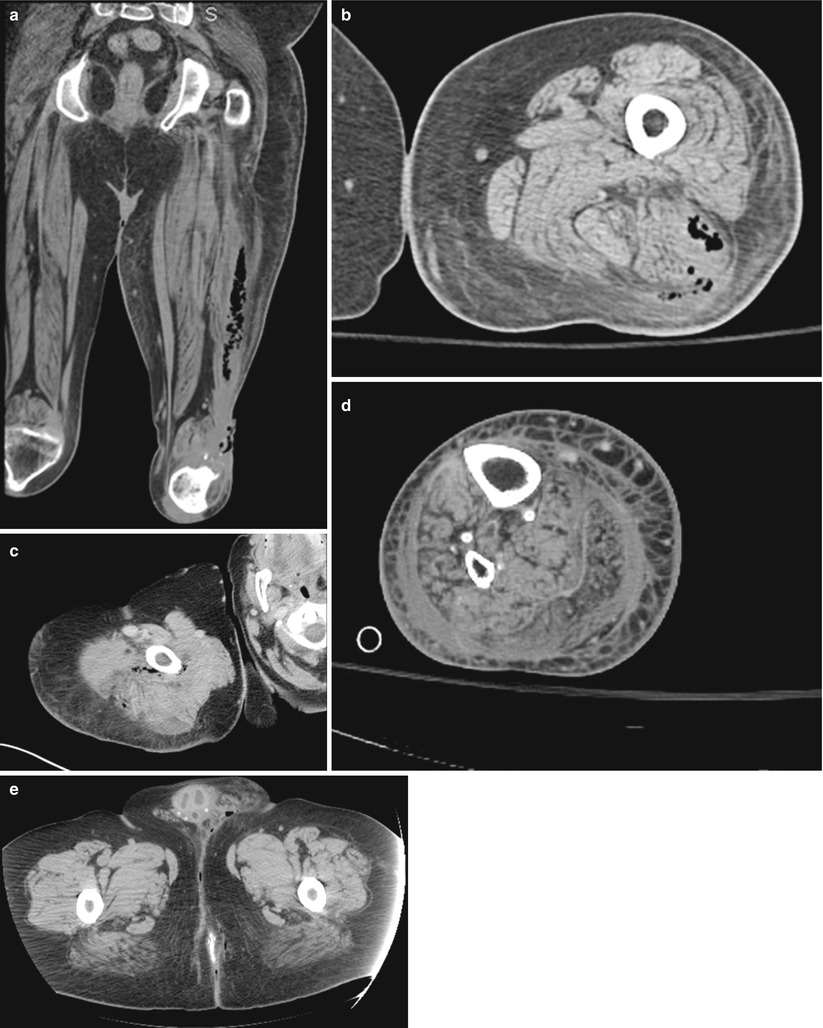
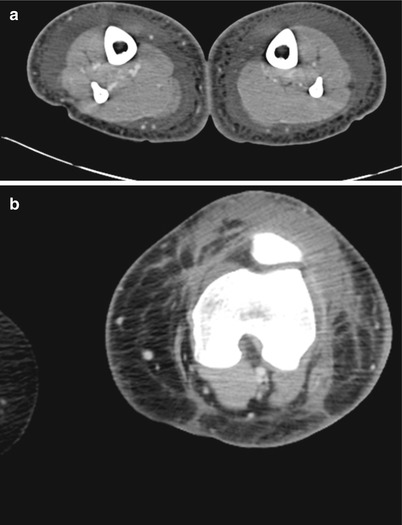
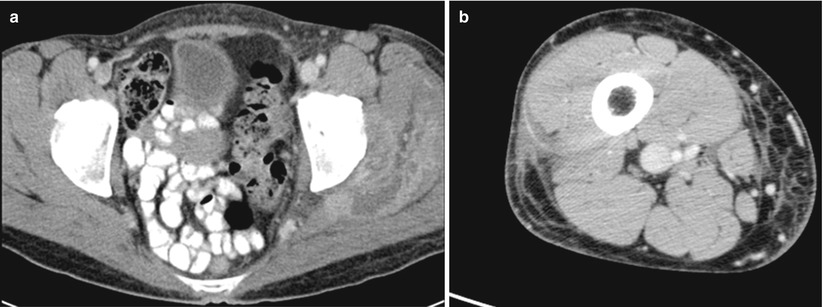

Fig. 17.7
NSTI computed tomography appearance. Author’s collection

Fig. 17.8
(a–e) Confirmed NSTI

Fig. 17.9
(a, b) Cellulitis – no NSTI

Fig. 17.10
(a, b) Abscess – no NSTI
Magnetic Resonance Imaging (MRI)
Without question, MRI is more sensitive than plain films, US, or CT in the detection of acute inflammation. In general, NSTI manifests as high signal intensity on T2-weighted images and as low-signal intensity on T1-weighted images [54]. Absence of Gd-DTPA contrast enhancement is strongly suggestive of tissue necrosis [55]. There are two main drawbacks of MRI. Firstly, this modality may be too sensitive. Falsely positive MRI scans may result in unnecessary surgical explorations for cellulitis. Secondly, MRI may not be immediately available in all institutions. Reliance on this modality for diagnosis may cause therapeutic delay. Routine application cannot be recommended.
Laboratory
No single laboratory value has sufficient accuracy to assist in the diagnosis of NSTI. Bacteremia is present in <30 % [12, 17, 25] and leukocytosis is nonspecific. In cases with equivocal physical exam and radiologic findings, a constellation of laboratory values may help distinguish the diagnosis of NSTI from cellulitis. Wong et al. have described a Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score to stratify patients into low-, moderate-, or high-risk categories using commonly ordered laboratory tests: C-reactive protein, WBC, hemoglobin, sodium, creatinine, and glucose [56]. According to the authors, at a cut-off LRINEC score of ≥6, their model has a PPV of 92 % and NPV of 96 %. While these results are impressive, one must apply the LRINEC with caution, as the LRINEC score may be less accurate in patients with multiple medical comorbidities and/or a blunted inflammatory response, both of which are present in the geriatric population.
A second group, Wall et al., have proposed a simple laboratory model based on two values: WBC > 15.4 and serum sodium < 135 [15]. These authors report that their model can distinguish NSTI from non-NSTI with a NPV of 99 %. Again, one must proceed with caution in the evaluation of the elderly patient as this model has not been validated in this population.
Surgical Exploration
It cannot be overemphasized that wide surgical debridement is the only effective therapy for NSTI and that time is of the utmost essence. Delay in surgical therapy has been repeatedly demonstrated to be associated with increased mortality and increased number of required debridements [29]. Even with severe hemodynamic and metabolic derangement, surgical exploration and debridement must proceed; physiologic resuscitation and correction is futile in the continued presence of infected, necrotic tissue. Because of the difficulty in establishing the diagnosis non-invasively, several authors have described bedside tests, including aspiration with Gram-stain [57], frozen section biopsy [58], and “the finger test.” The “finger test” is performed by making a small incision (2 cm) through the skin down to the fascia and bluntly probing the wound with a finger. A positive test (indicating NSTI) results if one can dissect the subcutaneous tissue off the fascia with minimal resistance [22]. Because of the possibility of sample bias and the potential delay in diagnosis and therapy, these lesser diagnostic operations are not recommended; in most instances the morbidity associated with delayed treatment of NSTI outweighs the morbidity of an exploratory incision in superficial skin and soft tissue infection.
The surgical approach at the first debridement should resemble a “search-and-destroy” mission. All frankly necrotic and infected tissue should be excised irrespective of anatomic or functional boundaries (Fig. 17.11). As this disease pays no respect to anatomic planes, neither should the treating surgeon hesitate to remove tissue when the patient’s life is at stake. At operation, affected tissue is easily recognized by lack of bleeding and lack of normal resistance to blunt dissection. In elderly patients, however, ease of tissue separation alone may not be a reliable indicator of infection.
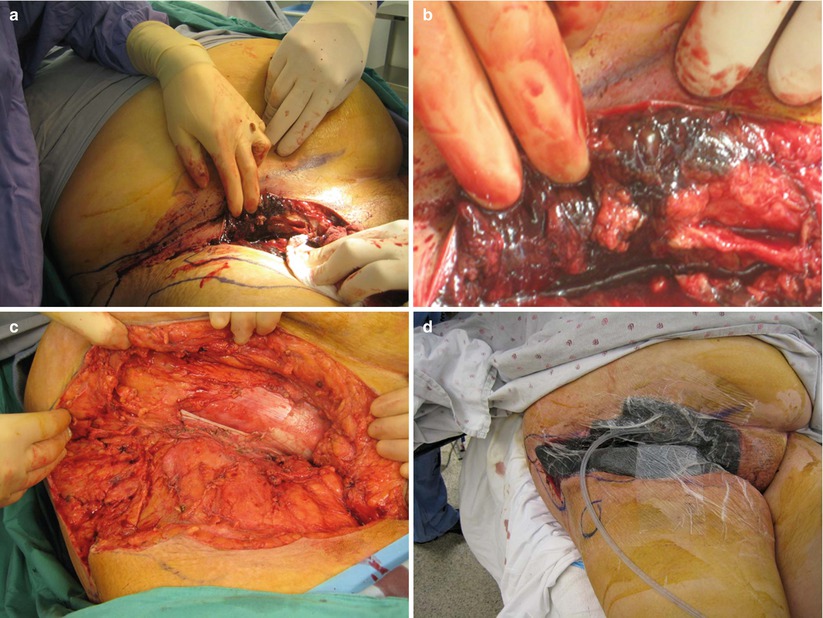

Fig. 17.11
NSTI intraoperative appearance. (a) Necrotic muscle and fat is evident; (b) necrotic muscle and fat is evident; (c) wound debrided to healthy, viable tissue; (d) VAC dressing on open wound. Author’s collection
A clinical caveat: finger dissection should be utilized with care in injection drug abusers as broken needle tips may reside in the subcutaneous tissues (Fig. 17.12). Necrotic fascia is noted to be discolored (grayish) and sometimes surrounded by a thin, foul-smelling “dishwater” liquid distinct from the garden variety creamy thick pus encountered in simple abscesses. Intraoperative aerobic and anaerobic cultures should be taken to aid in future targeted antibiotic therapy; these cultures should be taken from subcutaneous tissues, not from the skin surface or blister fluid. Tissue biopsies should be taken from the interface between necrotic and alive tissue for optimal diagnostic yield [38]. Skin should be debrided until brisk capillary dermal bleeding without epidermal discoloration is encountered. Large skin flaps may impede wound drainage and it may be prudent to excise skin with extensive underlining to facilitate wound care. Muscle and fascia should be debrided to healthy tissue with normal contractile function (in response to electrocautery).
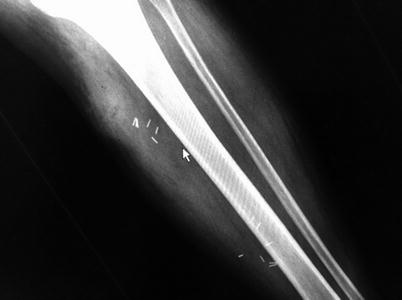

Fig. 17.12




Broken hypodermic needle tips embedded within the tissue. Author’s collection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree