1
Introduction
Diabetic foot disease (DFD) can present as diabetic neuropathy (DN), peripheral arterial disease (PAD), Charcot neuroarthropathy (CNA), diabetic foot ulcer, and osteomyelitis, also known as the diabetic foot syndrome. A higher prevalence of DFD is seen in males and those aged above 60 [ ]. Awareness of DFD among clinicians is important because it is a disease associated with high morbidity, mortality, and cost [ , ]. Poorly controlled DFD increases the likelihood of future lower limb amputation, with an amputation prevalence of 1.5% among people with diabetes [ , ]. DFD also accounts for more hospital admissions than any other diabetes complication [ ].
A good understanding of its risk factors is important in order to treat and prevent this devastating medical condition. In this chapter, we will discuss its prevalence, presentation, pathophysiology, treatment, and preventive measures that can be taken.
2
Diabetic neuropathy
Diabetic neuropathy (DN) is seen in up to 50% of all patients with diabetes [ ]. It may be painful (diabetic painful neuropathy, DPN) and negatively impact on mood, sleep and quality of life [ , ].
2.1
Pathophysiology
2.1.1
Sensory-motor neuropathy
The pathophysiology of DN is complex and multifactorial. Poorly controlled diabetes causes endoneurial ischemia and chronic hyperosmolarity, resulting in edema of nerve trunks [ ]. Distal polyneuropathy occurs resulting in sensory and motor deficits.
Sensory neuropathy manifestations include paresthesia and numbness of the distal extremity, initially distally then progressing proximally in a glove and stocking pattern. Symptoms tend to occur first in the feet and later involve the hands and upper limbs. As a result of the sensory loss, patients are less able to detect minor trauma such as abrasions.
Motor neuropathy manifests as weakness in the intrinsic and extrinsic muscles of the foot. This results in clawing and pes cavus, leading to greater peak plantar pressures [ ]. Weakness of the extrinsic and intrinsic muscles of the foot predisposes to ankle equinus and displacement of the plantar fat pads, respectively, further reducing the capacity of the tissue to reduce plantar pressure and thereby increasing the risk of plantar ulceration [ , ].
2.1.2
Cardiovascular risk factors
Although lower HbA1c levels are associated with lower prevalence of DN and vice versa, DN is also seen in patients with low HbA1c, indicating that there are also other risk factors at play [ ]. Cardiovascular risk factors such as hypertension and smoking have been associated with DN [ , ]. It may occur in these circumstances due to microvascular insufficiency and hypoxemia of the smaller arteries, including the vasa nervorum [ ].
2.2
Diagnosis
The diagnosis of DN is based on history and examination. Large and small nerve fiber function should be assessed using a 10 g monofilament on the plantar surfaces of the toes and metatarsal heads or checking the vibration sense by placing a 128 Hz tuning fork on the metatarsophalangeal joint and pain and temperature sensation, respectively. Joint proprioception, motor, and reflex arc of the lower limb should also be assessed.
2.3
Treatment
2.3.1
Optimization of diabetes control
Tight glycemic control limits progression of DN [ ]. Oral hypoglycemic agents, insulin, GLP-1 agonists, and SGLT 2 inhibitors are common pharmacological agents used in diabetes mellitus and have variable effects on HbA1c reduction.
2.3.2
Treatment of neuropathic pain
The American Academy of Neurology (AAN) published guidelines on the treatment of DPN with a focus on topical and oral medication, with moderate confidence grading given for gabapentinoids and serotonin and norepinephrine re-uptake inhibitors (SNRI) in improving pain compared to placebo.
Duloxetine, an SNRI, and pregabalin, a gabapentinoid, are approved by the US Food and Drug Administration and the European Medicines Agency in the treatment of DPN [ , ]. A combination therapy of duloxetine and pregabalin has been shown to be a safe, effective, and well-tolerated option for DPN in a multicenter, double-blinded parallel-group study [ ]. Combination treatment is recommended in patients with suboptimal pain control on monotherapy [ ].
Tricyclic antidepressants such as amitriptyline and topical medications such as capsaicin are more likely than placebo to improve pain; however, this was rated with low confidence by AAN.
Clinicians should not use opioids for the treatment of PDN, unless those described above have been tried and response to them has been suboptimal.
2.3
Conclusion
Poor glycemic control, duration of diabetes, and modifiable cardiovascular risk factors such as hypertension and smoking have been identified as risk factors for DN [ ]. It is essential that these risk factors are addressed.
In addressing neuropathic pain, combination therapy should be used if pain is sub-optimally controlled while on monotherapy.
3
Peripheral arterial disease
Peripheral arterial disease is a partial or complete obstruction of the peripheral arteries. It is more common in those with diabetes than those without, with an estimated prevalence of 20%, and is associated with a 15-fold higher risk of cardiovascular mortality [ , ]. PAD is more common in current smokers and those with longer duration of diabetes, higher hemoglobin A1c, hyperlipidemia, microalbuminuria, and hypertension [ ].
The condition is often asymptomatic. The most common symptom of PAD is intermittent claudication—lower extremity pain that develops with walking and relieved with rest.
3.1
Pathophysiology
The pathogenesis of PAD is atherosclerosis and thrombus formation. Diabetes is inherently associated with a hypercoagulable and proatherosclerotic state. Nitric oxide is an important mediator for vasodilation. Hyperglycemia blocks endothelial cell NO synthase, the enzyme that produces nitric oxide [ ]. Hyperglycemia also leads to increased production of proinflammatory reactive oxygen species [ ]. These two factors mediate the transformation of foam cells from leukocytes, precursors in atheroma formation in atherosclerosis. Poorly controlled diabetes also results in calcification of the tunica media layer of the artery, further hampering blood supply to the foot [ ].
More recent research suggests the pathogenesis of PAD to be more thrombi-dependent rather than atherosclerosis, with luminal occlusion and insignificant atherosclerosis commonly observed in patients with PAD [ ].
PAD is morphologically different between subjects with and without diabetes [ , ]. Vascular involvement in those with diabetes tends to be long (>10 cm), with more severe arterial disease seen in the profunda femoris and crural vessels than nondiabetic patients. This pattern of vascular involvement is distinct from those without diabetes where lesions tend to be short (<3 cm), with preferential involvement of the foot. As ischemia continues, dry gangrene may occur, particularly in the toes.
3.2
Diagnosis
Acute limb ischemia due to PAD is characterized by the sudden onset of lower extremity pain progressing to pallor, coolness, absence of palpable pulse, and finally paralysis. Findings of limb ischemia include absent or diminished pulses and reduced ankle brachial pressure index (ABPI).
Palpation of the femoral, popliteal, and pedal pulse (dorsalis pedis and posterior tibial) should be part of the routine assessment of the diabetic foot. In PAD, this may be absent or feeble. Of note, the dorsalis pedis and posterior tibial pulse may be absent in up to 8.1% and 2.0% of healthy individuals, respectively [ ].
The presence of ischemia can be measured objectively by measuring ABPI and should be performed on all patients with suspected PAD ( Table 20.1 ). This is calculated by dividing the systolic blood pressure (SBP) measured at the level of the ankle by the SBP measured in the brachial artery while in the supine position for 5 min. SBP should be obtained for the posterior tibial and dorsalis pedis arteries and higher value of the two should be recorded as the ankle pressure. SBP should be recorded in both arms with the higher value recorded as the brachial artery pressure. Patients with diabetes have arterial calcification, potentially resulting in falsely raised ankle systolic pressure and, therefore, falsely elevated ABPI [ ].
| ABPI measurement | Severity of PAD [ ] |
|---|---|
| 0.91–1.30 0.70–0.90 0.40–0.69 <0.40 | Normal Mild Moderate Severe |
Due to the high estimated prevalence of PAD in subjects with diabetes, the American Diabetes Association has recommended ABPI screening to be performed on patients aged above 50 with diabetes and further recommends consideration of screening in patients with diabetes aged less than 50 with other PAD such as smoking or diabetes duration for more than 10 years [ ].
The arterial doppler waveform should also be examined. The normal waveform is triphasic, consisting of a systolic forward flow, followed by an early diastolic flow reversal, and a late diastolic forward flow. As PAD progresses, the waveform becomes monophasic, consisting of a slow systolic forward flow into diastole without a reverse diastolic flow.
Contrast enhanced imaging (CT and MR) are accurate imaging modalities for evaluating disease severity and in planning revascularization. Relative contraindications to this include chronic kidney disease and contrast allergy.
Kidney dysfunction is a relative contraindication to contrast enhanced imaging given the theoretical risk of contrast induced nephropathy. There is no absolute value of kidney dysfunction in which the procedure will not be carried out as the decision to perform an angiogram should be made within a multidisciplinary team, with assessment of the potential risks and benefits gained from the investigation. If the patient has chronic kidney disease and is not on dialysis, carbon dioxide angiography instead of iodinated contrast may be used, as it is not excreted by the kidneys [ ].
3.3
Treatment
3.3.1
Medical optimization of risk factors
As PAD is associated with current smoking, poor diabetes control, hyperlipidemia, microalbuminuria, and hypertension, risk reduction by optimization of the treatment of these factors is advised.
3.3.2
Smoking cessation and pharmacological adjuncts
Patients should stop smoking as it increases reactive oxygen species, thereby promoting atherosclerosis [ ]. Nicotine replacement therapy (NRT) can help increase the chances of stopping smoking by 10% by reducing withdrawal symptoms associated with smoking cessation [ ]. NRT is available as chewing gum, nasal and oral sprays, inhalators, lozenges/tablets, and skin patches. Bupropion and varenicline are two other pharmacological therapies used in smoking cessation [ ].
3.3.3
Diet and exercise
Dietary intake plays a role in the development of atherosclerosis. Greater meat consumption was associated with higher incidence of PAD; meanwhile, those who followed a Mediterranean-diet pattern had a lower incidence of PAD [ , ].
There are two types of exercise programs—structured exercise program and community/home exercise program. The former is supervised and performed for a minimum of 30 min each time, at least 3 times a week for a minimum of 12 weeks. Training involves intermittent bouts of walking to moderate claudication, alternating with periods of rest. The latter is self-directed. Patients who did structured exercise programs had longer walking time and distance, but no effect was observed on mortality or lower limb amputation [ , ].
3.3.4
Optimization of diabetes control
Oral hypoglycemic agents and insulin are among the treatment armamentarium for diabetes and can result in reductions in HbA1c; however, positive cardiovascular outcome trials for such therapeutics are lacking. In contrast, guidelines have since been drawn following multiple cardiovascular outcome trials to highlight the preference for GLP-1 agonist and SGLT2 inhibitors in those with diabetes and atherosclerotic cardiovascular disease and those whose target is to promote weight loss [ ].
3.3.5
Statin
High-intensity statins such as atorvastatin 80 mg and rosuvastatin 40 mg help to reduce LDL-cholesterol by more than 40% and is associated with a lower risk of mortality and amputation compared to those prescribed low-to-moderate intensity statin [ , ]. Current professional guidelines recommend statin therapy for individuals with PAD, with target serum low-density lipoprotein cholesterol of less than 1.8 mmol/L or decreased by ≥ 50% if the initial LDL-C level is between 1.8 and 3.5 mmol/L [ , ].
Emerging therapies such as the addition of ezetimibe and/or PCSK-9 inhibitors to statin therapy have demonstrated an additional reduction in LDL-C level by up to 25% and 60%, respectively, with improved cardiovascular outcomes [ , ].
3.3.6
Antiplatelet and anticoagulation
Platelet adhesion is increased in PAD due to increased expression of P-selectin surface protein [ ]. Antiplatelet therapy therefore plays a key role in preventing complications. Aspirin and clopidogrel have been shown to reduce cardiovascular events in those with PAD, with current guidelines suggesting antiplatelet monotherapy in symptomatic lower extremity arterial disease or those who have undergone revascularization [ , ]. Clopidogrel is the preferred antiplatelet drug [ ].
As PAD is also driven by thrombus formation, anticoagulation reduces complications from PAD and cardiovascular mortality [ ]. Although anticoagulation therapy should be started only if there is a concomitant indication, low-dose anticoagulation with rivaroxaban 2.5 mg twice daily in combination with aspirin may be used and is licensed in prophylaxis of atherothrombotic events in patients with symptomatic peripheral artery disease. In the multicenter, double-blind, placebo-controlled COMPASS trial, those assigned to low dose rivaroxaban plus aspirin had an incidence of reduced limb ischemia needing intervention or major lower limb amputation [ ]. They also had lower stroke and cardiovascular mortality than aspirin only group. However, patients should be counseled about the risk of bleeding given the higher major bleeding events that occurred in the combination group. This cardiovascular benefit was not demonstrated in those with PAD taking warfarin and aspirin [ ].
3.3.7
Hypertension and microalbuminuria
Hypertension and microalbuminuria are associated with PAD and are risk factors associated with increased all-cause mortality [ , ]. ACE inhibitors slow the progression of microalbuminuria, control hypertension, and reduce cardiovascular mortality [ ]. Guidelines recommend a target blood pressure in patients with PAD of 140/90 mmHg with ACE inhibitors advocated as first line therapy [ , ].
3.3.8
Revascularization and limb amputation
The indications for limb revascularization are intermittent claudication, rest pain, or tissue necrosis refractory to medical treatment. Revascularization may occur via endovascular or open procedure. The two approaches are not mutually exclusive and may be combined. Balloon angioplasty is the most commonly used interventional radiology technique in revascularization. It may be more appropriate in patients with focal stenosis affecting larger proximal vessels. The most important factor affecting revascularization success is the number of patent arteries post procedure [ ]. Restenosis rate after angioplasty of extensive infrapopliteal arterial disease is high and occurs early after treatment, so follow up is essential in all postprocedural patients. Compared to endovascular revascularization, open procedures have been successfully carried out for all lesions and tend to have greater durability. Aorto-iliac and crural disease are traditionally treated with aorto-femoral bypass and bypass to the distal vessels using the saphenous vein, respectively.
Major amputation in the neuroischemic foot is necessary when there is overwhelming life-threatening infection. Lower limb amputation is associated with subsequent amputation, reduced functional status, and may be associated with increased mortality [ ].
3.4
Conclusion
PAD is associated with increased cardiovascular mortality. Clinical examination and ABPI measurement help to detect PAD by the bedside. Revascularization and medical optimization of risk factors like diabetes and dyslipidemia are cornerstones in PAD management.
4
Charcot foot/Charcot neuroarthropathy
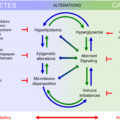
One of the most challenging complications of diabetic foot to manage is Charcot foot, also known as CNA. It is characterized by joint and bone destruction, and ligamentous laxity in the bones and joints in the feet. It was first described by Jean-Martin Charcot in 1868 in patients with syphilis and neuropathy. Diabetes is the leading cause of Charcot foot in the Western world.
4.1
Pathophysiology
Neuropathy, excess bone resorption, fractures, and diabetes are all contributors to the development of Charcot foot.
4.1.1
Sensory/motor and autonomic neuropathy
Sensory-motor DN manifest as paresthesia, numbness, and weakness in the intrinsic and extrinsic muscles of the foot which predispose to plantar ulceration.
Sympathetic denervation results in decreased sweating and increased bone resorption [ ]. It also leads to vasodilation and arteriovenous shunting, causing peripheral edema. Decreased sweating predisposes to dry skin which increases risk of microbial infection.
4.1.2
Excess bone resorption
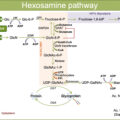
Bone remodeling is governed by bone-forming osteoblast and bone-resorbing osteoclast. Osteoblasts produce RANK ligand (RANKL) and osteoprotegerin (OPG). RANKL binds to the RANK receptor on osteoclast, activating osteoclast activity and therefore stimulates bone resorption. RANKL is also produced by T cells during inflammation, thereby increasing bone resorption. OPG, on the other hand, inhibits osteoclast activation by acting as a decoy receptor for RANKL.
Other mediators in bone remodeling include cytokines. Proinflammatory cytokines such as TNF-α and IL-1β augment osteoclast activity by directly stimulating osteoclast precursor cells and mature osteoclasts.
In Charcot, there is an excess of osteoclast activity due to increased activity of RANKL and proinflammatory mediators [ ].
4.1.3
Trauma/fractures/surgery
Ankle/foot trauma or fractures or surgery on the background of neuropathy may trigger Charcot through the release of proinflammatory cytokines that augment osteoclast activity [ ].
4.1.4
Diabetes
The formation of advanced glycation end products (AGEs) in uncontrolled diabetes induces apoptosis of osteoblasts and result in defective collagen formation [ ]. This results in osteopenia with an increased risk of insufficiency fractures of the ankle and/or metatarsals.
4.2
Diagnosis
Orthopedic surgeon Sidney N. Eichenholtz classified stages of Charcot arthropathy based on a mixed cohort of patients with Charcot arthropathy due to diabetes, syphilis, leprosy, alcoholism, and syringomyelia ( Table 20.2 ) [ ].
| Stage | Clinical findings | Radiological findings | Nonsurgical treatment |
|---|---|---|---|
| 0 (Prodromal) | Warm, swollen, and erythematous | Normal | Total contact cast |
| 1 (Development) | Warm, swollen, and erythematous | Joint subluxation or dislocation, osteopenia | Total contact cast |
| 2 (Coalescent) | Decrease in warmth, swelling, and erythema | Sclerosis and fusion of fragments | Removable cast walker |
| 3 (Reconstructive) | Resolution of warmth, swelling, and erythema | Joint arthrosis | Extra depth shoes with custom molded insole |
Early stage of Charcot foot is characterized by a unilateral warm, swollen red foot. The affected foot is usually more than 2°C warmer than the nonaffected foot. As joint destruction progresses, ulcers and deformities may ensue, including rocker bottom foot, caused by collapse of the bones that form the medial arch.
Charcot foot tends to occur unilaterally; however, bilateral involvement has been reported to occur in up to 39.3% of cases. The majority of this population have had diabetes for more than 10 years, with a mean age of onset being 57 years. The majority of Charcot foot occurs in the midfoot [ ].
X-ray may be normal in the early stages of the disease. Imaging modalities like MRI and PET have enabled early detection of the disease by demonstrating bone marrow edema.
It is important to differentiate between Charcot foot and other differentials such as cellulitis and deep vein thrombosis because delay of immediate treatment perpetuates further joint destruction and disorganization resulting in higher risk of complications.
Anatomical patterns of joint destruction: Five patterns of joint destruction have been described by Sanders and Frykberg [ ].
Type 1 (15%): Affecting the forefoot—phalanges, interphalangeal joint, metatarsal, and metatarsophalangeal joints.
Type 2 (40%): Tarsometatarsal joint (Lisfranc)
Type 3 (30%): Talonavicular and calcaneocuboid joint (Chopart)
Type 4 (10%): Ankle joint
Type 5 (5%): Calcaneus
Midfoot involvement accounts for the majority of Charcot foot cases, and these can usually be successfully managed by nonsurgical means. Involvement of the hindfoot is less common, and such deformities are poorly tolerated, hence are more likely to require surgical intervention.
Staging helps guide the management, as those in stage 1 require strict offloading with a total contact cast—a nonremovable and knee-high molded cast. As inflammation subsides, patients are transitioned into removable cast walkers. When all signs of inflammation have resolved, patients are transitioned into extra-depth shoes with custom molded insoles to prevent ulcer formation.
4.3
Management
Patients with suspected or confirmed CNA should be immediately referred to a multidisciplinary diabetic foot clinic because early intervention could arrest disease activity.
4.3.1
Offloading
Offloading using total contact cast and removable cast is the cornerstone to managing Charcot foot as it reduces forefoot peak pressures by up to 90% [ ]. Patients are advised to use crutches or wheelchairs to avoid weight bearing on the affected side.
4.3.2
Total contact cast
The International Working Group on the Diabetic Foot (IWGDF) guidelines for diabetic neuropathic ulcers recommends nonremovable and knee-high total contact casting as the gold standard of care. Total Contact Cast (TCC) consists of a minimally padded molded cast that maintains close contact with the plantar aspect of the foot and lower leg with the aim of redistributing the elevated plantar pressures seen in Charcot foot and also eliminating ankle joint motion.
The affected limb is immobilized in an irremovable TCC in the development stage of Charcot foot (stage 1), with the aim of dampening the inflammatory process. Initially, the TCC is replaced at 3 days, then it is checked weekly. Patients should be monitored for any complications that can arise due to casting such as ulcers.
TCC is continued until inflammation subsides, with radiographic signs of healing or resolution of erythema, swelling, and warmth as seen in the reconstructive stage of Charcot foot (stage 3). Resolution of warmth is defined when the temperature differential is less than 2°C between the affected and nonaffected foot using infrared skin thermometry. Globally, there are discrepancies regarding the duration of TCC treatment for resolution of acute Charcot foot, from a few months to a year [ ]. TCC is relatively contraindicated in patients with osteomyelitis, active wound infection, and PAD.
Removable cast walkers offload the forefoot and midfoot but have the added benefit of being able to be unlocked to allow for wound inspection. They may contain bead-filled inserts (as in VACOcast) or be inflatable (as in air cast boot). It is important that the beads are in the right place or that the boot is fully inflated, so that the cast boot can prevent movement of the foot and ankle.
4.3.3
Joint arthrosis
Compared to a cast boot, the Charcot Restraint Orthotic Walker (CROW) is a custom rigid boot designed to manage hindfoot Charcot foot. It consists of a fully enclosed ankle and foot orthotic made of fiberglass material with a custom rocker bottom sole. The close fitting provides more total contact offloading than a cast boot, and any marked deformity can be accommodated to reduce risk of rubbing in a cast boot.
4.3.4
Extra depth shoes with custom molded insole
Compared to TCC, they may provide more comfort, however, may be associated with poorer healing rates because patients have a tendency to take them off [ ]. These tend to be used in the coalescent phase of the Charcot foot.
4.3.5
Extra depth shoes
Extra depth shoes that come with custom molded insoles are used to prevent the occurrence of ulcers. They are used in the reconstructive stage of Charcot foot (stage 3).
4.3.6
Orthopedic interventions
The primary goal of surgical intervention in CNA is to reduce pressure and control infection where present. Surgical intervention during the prodromal and development phase of CNA is ill advised as excessive inflammation during this phase impedes bone healing and fixation due to ongoing osteoclastic activity. Rates of complications among the cohort are high due to comorbidities like diabetes, neuropathy, and PAD.
4.3.7
Bone resection
Bone resection is performed in cases where osteomyelitis is resistant to antimicrobial therapy.
4.3.8
Exostectomy
Bony prominences are most common in the midfoot and develop when the tarsal bones dislocate during the development phase of CNA. Exostectomy should be used to relieve bony pressure when nonsurgical treatments fail to achieve ulcer healing.
4.3.9
Lengthening of the Achilles tendon
Patients with ankle equinus deformity are more likely to have a longer duration of diabetes than those without, resulting in higher peak plantar pressures. Addressing this deformity reduces forefoot pressure and therefore potentially reduces risk of ulceration and amputation [ ]. Combining Achilles tendon lengthening with a nonremovable cast had higher forefoot ulcer healing rates than nonremovable cast alone in two small trials [ ].
4.3.10
Arthrodesis
The aim of arthrodesis is to provide joint stability. It is used in patients with joint instability, pain or recurrent ulcerations who fail nonoperative treatment. Deformities may be corrected in a single stage or multistage process by means of internal and/or external fixation, with a preference for external fixation in the presence of ongoing soft tissue/bone infection. Patients should remain nonweight bearing to allow for osseous healing. The specific type of arthrodesis procedure that is performed and duration of nonweight bearing status is guided by the location of the involved joint and clinical signs of healing.
An overall fusion rate of 86% has been reported, with limb salvage rates of up to 96% reported in those definitively managed with external fixation of the midfoot and hindfoot [ ]. Unfortunately, the complication rates for CNA reconstruction are high—common complications include incomplete bone union, malunion, infection, and hardware failure [ ].
4.3.11
Amputation
Amputation is the last resort for those who fail medical and other surgical interventions, especially in those with refractory infection. Indications where antimicrobial treatment alone is unlikely to successfully control infection, include crepitus on clinical examination, and purplish discoloration of the skin indicating subcutaneous necrosis [ ].
4.4
Medical interventions
While pharmacotherapy that alters bone turnover is an attractive target, unfortunately, there is little evidence to suggest it promotes healing of the Charcot foot.
4.4.1
Bisphosphonate
Bisphosphonates (BPP) are used to treat bone and calcium disorders such as osteoporosis and primary hyperparathyroidism. BPPs work by attaching to remodeled bone. Osteoclasts then migrate to these areas and ingest it, releasing cytotoxic analogs of ATP which leads to apoptosis of osteoclast.
Intravenous or oral BPP on patients with diabetic CNA has been shown in a few small-scale trials to result in a fall of temperature differential between the feet and a decrease in alkaline phosphatase activity, a marker for bone turnover [ , ].
The consensus report on the Charcot foot says that there is limited evidence to support its use and that larger well powered randomized controlled trials are required [ ].
4.4.2
Calcitonin
Calcitonin is a peptide secreted by the parafollicular cells of the thyroid gland. It binds to calcitonin receptors on osteoclasts to inhibit its action and reduces bone resorption markers [ ]. Larger trials are needed to assess its role in patients with CNA and whether this translates to improved outcomes.
4.4.3
TNF-α and IL-1β blockers
Pro-inflammatory cytokines such as TNF-α and IL-1β augment osteoclast activity, with increased levels observed in Charcot arthropathy. TNF-α inhibitors such as etanercept and IL-1β blocker such as anakinra have been shown to slow disease progression of inflammatory arthritis. However, its use in CNA remains to be proven.
4.4.4
RANKL inhibitors
As mentioned previously, RANK ligand (RANKL) produced by osteoblasts activates osteoclast activity and therefore stimulates bone resorption. Denosumab is a monoclonal antibody against RANKL and is licensed for use in treating osteoporosis in postmenopausal women and men at increased risk of fractures. Unfortunately, its use in CNA remains to be elucidated.
4.4.5
Parathyroid hormone
Parathyroid hormone (PTH) is an anabolic agent that stimulates osteoblast and has been used successfully in a few cases of nonunion after arthrodesis operation for Charcot arthropathy [ ]. Its use is contraindicated in skeletal malignancies. However, a recent double-blinded placebo-controlled trial failed to demonstrate any added benefit of PTH to resolution of CAN [ ].
4.5
Conclusion
Charcot neuroarthropathy is one of the most challenging complications of diabetic foot to manage. Clinical and radiological findings help determine the phase of CNA and form of nonsurgical management. Total contact cast is the gold standard treatment for CNA. Orthopedic interventions help to further alleviate plantar pressure. Unfortunately, there is a dearth of robust evidence for pharmacotherapeutics in halting Charcot foot progression.
5
Diabetic foot ulcer
Diabetic foot ulcer (DFU) is defined as a full thickness skin defect distal to the malleoli. They most commonly occur on the pulp of the hallux and beneath the first metatarsal. Larger size, depth, and longer duration of DFU are associated with longer time to heal and poorer prognosis [ ]. DFU represents a significant global challenge. Approximately, 18%–34% of individuals with diabetes will develop a DFU within their lifetime [ ].
5.1
Pathophysiology
Diabetic foot ulcers form due to trauma that happens in the presence of neuropathy and/or ischemia. They may either be infected or noninfected.
The neuropathic foot is warm, well perfused with dry and shiny skin—changes also seen in CNA. Sensory-motor neuropathy results in greater peak plantar pressures typically under the metatarsal heads or on the plantar aspect of the toes and therefore increase the risk of callus formation. If allowed to become too thick, the callus will press on soft tissues and cause ulceration.
The ischemic foot is cool and pulseless—changes described in PAD. All patients with DFUs should have ABPI and arterial Doppler waveforms performed to rule out the presence of PAD.
Signs of wound infection include malodor, purulent discharge, and yellowish slough at the base of the ulcer as opposed to nonodorous healthy pink granulation tissue. Most mild diabetic foot infections are caused only by aerobic Gram-positive cocci, predominantly Staphylococcus aureus . In moderate to severe diabetic foot infections, the causative organism is usually polymicrobial with a mixture of Gram-positive cocci, Gram-negative rod (e.g., E . coli ) and nonfermentive Gram-negative rod (e.g., Pseudomonas). Antimicrobial resistant pathogen (e.g., MRSA) should be suspected in patients with severe infection, risk factors for MRSA infection (such as previous antibiotic therapy and frequent hospitalization) or have evidence of colonization of this organism elsewhere.
5.2
Classification
There are several classification systems for diabetic foot ulcers. The two described here are the University of Texas Staging System ( Table 20.3 ) [ ] and Infectious Diseases Society of America (IDSA) Infection Severity ( Table 20.4 ) [ ]. There is no consensus on which classification is superior.