Fig. 11.1
The four temperaments—(from left to right) choleric, melancholic, sanguine and phlegmatic— on the wall of a house at the corner of Am Dornbusch and Eschersheimer Landstraße in Dornbusch, Frankfurt am Main, Germany. Artist unknown
Anecdotal reports of splenic surgery began to emerge in the sixteenth century (Zacarello, 1549). Until modern times, however, removal of the spleen usually resulted in death. Most splenectomies were done in patients who had undergone penetrating trauma and with the spleen protruding from the wound. The first elective splenectomy was carried out for portal hypertension in 1826 by Quittenbaum: the patient died. In 1966, Bryant was the first to attempt splenectomy for leukemia. Over the following 15 years, 14 splenectomies were attempted as therapy for leukemia: none of the patients survived. By 1877, only 50 splenectomies had ever been undertaken, with a mortality rate of >0%. In 1908, Johnston reported a mortality of 87.7% in a review of splenectomy for hematologic disorders [1].
With the increased use of automobiles, in the twentieth century splenectomy became more common and the morality rate dropped rapidly. In a 1920 report of the experience with splenectomy of the Mayo Clinic, a mortality rate of 11% was documented [2]. Nowadays, the largest splenectomy series reported mortality rates of <1%.
Moreover, since the first laparoscopic splenectomy (LS) described by Delaître in 1991 [3], numerous case series have shown the feasibility of LS for a large variety of benign and malignant hematologic diseases. Excluding trauma, benign hematologic diseases are now the most common indication for splenectomy. Splenectomy may be indicated as a diagnostic tool and surgical staging or for palliation in patients with malignant hematologic disease. Patients with malignant hematologic diseases are more likely to have massively enlarged spleens (>1,000 g), resulting in significant discomfort and pain. Splenectomy can also provide relief to patients with symptomatic splenomegaly.
Anatomical and Functional Considerations
Consisting of an encapsulated mass of vascular and lymphoid tissue, the spleen is the largest reticuloendothelial organ in the body. The abdominal surface of the diaphragm separates the spleen from the lower left lung and pleura and the ninth to eleventh ribs. The visceral surface faces the abdominal cavity and contains gastric, colic, renal, and pancreatic impressions (Fig. 11.2).
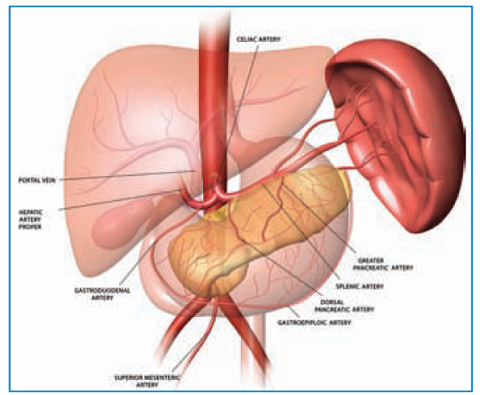

Fig. 11.2
Three-dimensional drawing of the normal anatomy in the upper abdomen shows the main splenic artery and its branches. Reproduced from [25] with permission from the Radiological Society of North America
Size and Dimensions
Spleen size and weight vary with age and pathologic conditions. The average adult spleen is 7 cm to 11 cm in length and weighs 150 g (range, 70 g to 250 g). Splenomegaly is described variably and the surgical literature reflects a lack of consensus. Spleen size is expressed in terms of the maximum interpole length and is generally classified into three categories: normal size (<11 cm), moderate splenomegaly (11 cm to 20 cm or >500 g in weight) and severe or massive splenomegaly (>20 cm or >1,000 g in weight) [4].
Anatomical Anomalies
The most common anomaly of splenic embryology is the accessory spleen (AS). Present in ≤20% of the population, one or more ASs may also occur in ≤30% of patients with hematologic disease. More than 80% of ASs are found in the region of the splenic hilum and vascular pedicle. Other locations for ASs (in descending order of frequency) are the gastrocolic ligament, pancreas tail, greater omentum, greater curve of the stomach, splenocolic ligament, small and large bowel mesentery, left broad ligament in women, and left spermatic cord in men [5]. For these reasons, ASs should always be considered, even if the hematologic significance of the presence of AS and the impact of their removal on results are not clear. The role of ASs in failed splenectomy has been studied extensively, and the prevalence of residual splenic tissue (as detected by post-splenectomy scintigraphy) reaches .48%, even if the efficacy of accessory splenectomy varies from 27% to 75% [6].
Surprisingly, also after laparoscopic splenectomy, residual splenic tissue was noted in 50% of patients, [7] even if it could be related to the surgical approach. The problem of ASs can be managed by careful videoscopic examination of the abdominal cavity during splenectomy, whereas the use of preoperative imaging methods for the detection of ASs is limited by the insufficient sensitivity of these methods [8].
Relationship with the Pancreas
The relationship of the pancreas to the spleen also has important clinical implications. In one cadaveric anatomic series, the tail of the pancreas was demonstrated to lie within 1 cm of the splenic hilum in 75% of cases and in 30% to the border with the spleen [9]. Considering that injuries to the pancreatic tail can cause important postoperative complications such as pancreatitis or pancreatic fistulas, the pancreatic tail should be carefully visualized and dissected from the spleen, avoiding damage by electrocautery or a linear stapler.
Vascular Considerations
The main arterial blood supply occurs through the splenic artery (the longest and most tortuous of the three main branches of the celiac artery). The spleen also receives some of its blood supply from the short gastric vessels that branch from the left gastroepiploic artery running within the gastrosplenic ligament. The splenic artery can be characterized by the pattern of its terminal branches. The distributed type of splenic artery is the most common (70%) and is distinguished by a short trunk with many long branches entering over three-fourths of the medial surface of the spleen. The less common magistral type of splenic artery (30%) has a long main trunk dividing near the hilum into short terminal branches, and these enter over 25% to 30% of the medial surface of the spleen [10]. Early identification of the type of splenic blood supply can help the surgeon to estimate how difficult the splenectomy is likely to be.
Outside the spleen, the arteries also frequently form transverse anastomoses with each other and, as a consequence, attempts to close a splenic-artery branch by clips or embolization, if undertaken proximal to these anastomosis, may fail to devascularize the corresponding splenic segment. Before division of the splenic trunk, it usually gives few small branches (the most important is the pancreatica magna) to the tail of the pancreas. Occlusion or section of these branches could result in pancreatitis. The main venous drainage is through the splenic vein, which joins the superior mesenteric vein behind the neck of the pancreas to form the portal vein.
Spleen Function
The spleen combines the innate and adaptive immune system in a uniquely organized way. The structure of the spleen enables it to remove older erythrocytes from the circulation and leads to the efficient removal of blood-borne microorganisms and cellular debris. This function, in combination with a highly organized lymphoid compartment, makes the spleen the most important organ for antibacterial and antifungal immune reactivity [11].
The spleen, which has important hematopoietic functions during early fetal development (until the fifth month), has no significant hematopoietic function remaining in adults, but continues to function as a “sophisticated filter” because of the unique circulatory system and lymphoid organization, and it has blood-cell monitoring and management functions as well as important immune functions throughout life. However, under certain pathological conditions (such as myelodysplasia), the spleen can reacquire its hematopoietic function.
Removal of the spleen results in loss of immunologic and filtering functions. It is well established that, after splenectomy, patients are at a significantly higher risk for overwhelming post-splenectomy nfection (OPSI) with fulminant bacteremia, pneumonia, or meningitis, as compared with those with normal splenic function. Asplenic subjects have defective activation of complement by the alternative pathway, leaving them more susceptible to infection. Asplenic patients also have a normal response to re-immunization to an antigen first encountered before splenectomy, but do not have an optimal response to exposure to new antigens, especially if the antigen is administered intravenously.
The spleen is a major site of production of opsonins (molecules that target an antigen for an immune response), properdin (important for initiation of the alternative pathway of complement activation) and tuftsin (a tetrapeptide that enhances the phagocytic activity of polymorphonuclear leukocytes and mononuclear phagocytes). Removal of the spleen results in decreased serum levels of these factors. As a result, neutrophil function is decreased in asplenic patients, and the defect appears to be related to the absence of circulating mediators [12].
For these reasons, it is suggested that splenectomized persons receive the following vaccinations (ideally before planned splenectomy):
Pneumococcal polysaccharide vaccine (not before 2 years of age);
Hemophilus influenzae type B vaccine (especially if not received in childhood);
Meningococcal conjugate vaccine (especially if not received in adolescence);
Influenza vaccine, every winter (to help prevent getting secondary bacterial infection).
Surgical Considerations
Preoperative Evaluation
Currently, we consider all patients evaluated for elective splenectomy to be potential candidates for laparoscopic splenectomy (Fig. 11.3). Large case series and non-randomized comparative trials have consistently reported better outcomes from laparoscopic splenectomy than from open splenectomy. Although operating times were longer among patients who underwent LS, the duration of hospital stay, transfusion requirements, and morbidity and mortality were reduced [13].
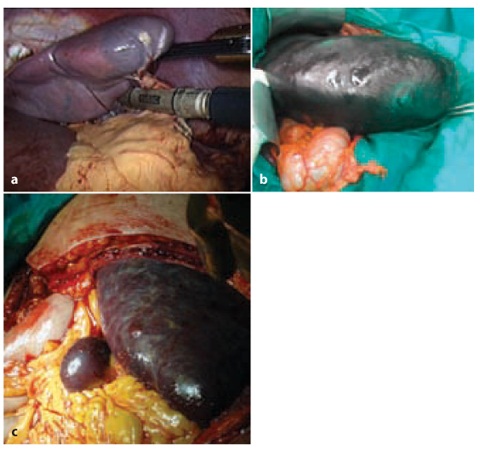

Fig. 11.3
Different surgical approaches to splenectomy for splenomegaly: a laparoscopy, b–c laparotomy
Contraindications to a laparoscopic approach include severe portal hypertension, uncorrectable coagulopathy, severe ascites, and most traumatic injuries to the spleen. Extreme splenomegaly also remains a relative contraindication.
Because most patients scheduled for splenectomy have a hematologic disorder, a multidisciplinary treatment protocol should be adapted to each patient, in particular about the use of corticosteroids, gamma-globulins, and platelet transfusion.
Patients undergoing elective splenectomy should be vaccinated preoperatively (or within 30–40 days postoperatively) with pneumococcal, meningococcal, and Haemophilus vaccines. Low-molecular-weight heparin (LMWH) prophylaxis for thromboembolism and antibiotics are administered according to standard guidelines (and if there are no hematologic contraindications). Bowel preparation is not routinely necessary. Patients need to be given all the information about the consequences of the asplenic state.
Massive Splenomegaly
The literature reports that, with sufficient experience, even massive spleens can be removed safely using minimally invasive methods [14]. In any case, rather than the absolute size of the spleen, the relationship between the same and amplitude of the laparoscopic chamber is more important [15]. Knowledge of anatomy is essential for preparation of the splenic hilum and liberation of the organ. Moreover, preoperative embolization of the splenic artery and the hand-assisted method allowed extension of the laparoscopic indications for massive splenomegaly [16]. In the process of hand-assisted laparoscopic splenectomy for splenomegaly, tactile senses may help to identify dissection planes, to define ASs, and to prevent splenic capsular injury by trocars and instruments. The hand may function as a retractor to hold the stomach, colon and pancreas moderately while holding the spleen laterally simultaneously. Furthermore, dissection of the splenic hilum could be easier with the tactile sense of the hand combined with a laparoscopic dissector in the other hand even in the setting of a spleen with dense adhesion.
However, because of the larger-sized spleen that narrows the operative space, exposure is limited and the manipulation difficult, LS becomes more technically challenging. The hypervascularization and dense adhesion around the spleen also hampers the procedure. Moreover, once the dissection is completed, extraction of the giant spleen with a totally laparoscopic approach by placing into a retrieval bag followed by morcellation can be difficult, and the procedure time can be longer. LS for splenomegaly has been associated with longer operating time, more blood loss, and higher intraoperative and postoperative complication rates than LS for normal-sized spleens,and with a higher conversion rate [17].
Extraction of Specimens
Spleens removed by an anterior laparoscopic approach are extracted through the umbilical trocar site after fragmentation in a plastic bag. It is rarely necessary to enlarge the incision by >2–3 cm. If the lateral approach is undertaken, extraction is more readily carried out through one of the ports situated anteriorly. This extraction site also requires little or no enlargement. On occasion, for spleens longer than 20 cm, a Pfannenstiel incision should be made or the spleen can be readily extracted through the accessory incision used for hand assistance during hand-assisted laparoscopic surgery.
Special mention should be made for laparoscopic splenectomy in patients with malignant disease. If lymphoma (Hodgking or non-Hodgkin) is suspected, neither preoperative splenic artery embolization nor spleen fragmentation should be performed, for avoid compromising the histological diagnosis. In practice, however, the histological diagnosis was made preoperatively in the majority of the patients, then this measure may not be necessary.
Percutaneous Image-guided Biopsies
Historically, image-guided percutaneous biopsy of the spleen has been approached with trepidation by radiologists because of concerns regarding accessibility and the risk of hemorrhage. This reluctance may be related to an early report of a high rate of major complications (13%) for percutaneous biopsy of the spleen performed with a 14-G needle [18]. Several more recent studies have reported much lower complication rates with smaller needle diameters (≤18 G gauge), similar to that reported for kidney or liver biopsies [19]. Diseases that commonly affect the spleen can pose a diagnostic challenge to the clinician, radiologist, and pathologist. The reported diagnostic accuracy of splenic biopsy varies, ranging between 84% and 90% [20].
For cases in which the spleen is the only abnormal or most accessible organ for biopsy and a tissue diagnosis is required, the use of image-guided percutaneous biopsy of the spleen could be considered a safe alternative to splenectomy with a high overall diagnostic accuracy (but only in experienced hands) [21].
Preoperative Embolization of the Splenic Artery
Preoperative embolization of the splenic artery can reduce the operating time and decrease intraoperative blood loss when compared with laparoscopic or open splenectomy alone [22]. However, besides higher costs, radiation exposure for the patient and the unavailability of the procedure in each hospital, there are other problems related to the angiographic embolization, in particular the higher risk of portal-vein thrombosis (≈15%) and the pain related to ischemic damage [23]. In fact, ≈45% of patients report some degree of pain [24].
As the use of splenic arterial interventions increases in interventional radiology practice, clinicians must be familiar with splenic vascular anatomy, the indications and contraindications for carrying out interventional procedures, the technical considerations involved, and the potential use of other interventional procedures (such as radiofrequency ablation) in combination with splenic arterial interventions. Familiarity with the complications that may result from these interventional procedures, including abscess formation and pancreatitis, is also important [25].
Splenectomy for Benign Hematologic Disorders
Splenectomy continues to find common therapeutic indications for hematologic disorders in which the spleen has a pathologic role (Table 11.1). Hematologic disorders can be categorized by various criteria, including the origin of the disorder (splenic, peripheral blood cell, bone marrow, genetic) or cell-line abnormality (platelets, red blood cells (RBCs), white blood cells (WBCs), and bone marrow). Therapeutic splenectomy rarely cures the underlying hematologic disease but, in many of these disorders, splenectomy can significantly improve the pathologic effects of splenic sequestration and symptomatic splenomegaly, can correct the hematologic abnormality, and can aid in the diagnosis and staging. Removal of the spleen can play an important part in reducing the morbidity of hematologic conditions [26, 27].
Table 11.1
Indication and response to splenectomy in hematologic diseases
Disease/Condition | Indications for Splenectomy | Response to Splenectomy |
|---|---|---|
Benign Diseases | ||
Idiopathic thrombocytopenic purpura | Failure of medical therapy, recurrent disease | 75–85% rate of long-term response |
Thrombotic thrombocytopenic purpura | Excessive plasma exchange requirement | 40% curative rate |
Hereditary spherocytosis | Hemolytic anemia, recurrent transfusions, intractable transfusions, intractable leg ulcers | Improves or eliminates anemia |
Pyruvate kinase deficiency | Only in severe cases, recurrent transfusions | Decreased transfusion requirement, palliative only |
G6PD | deficiency None | — |
Warm-antibody autoimmune hemolytic anemia | Failure of medical (corticosteroid) therapy | 60–80% response rate, recurrences common |
Sickle cell disease | History of acute sequestration crisis, splenic symptoms or infarction | Palliative, variable response |
Thalassemia | Excessive transfusion requirements, symptomatic splenomegaly or infarction | Diminished transfusion requirements, relief of symptoms |
Felty’s syndrome | Neutropenia | 80% durable response rate |
Gaucher’s disease | Hypersplenism | Improves cytopenias; does not correct underlying disease |
Niemann-Pick disease | Symptomatic splenomegaly | Improves symptoms; does not correct underlying disease |
Amyloidosis | Symptomatic splenomegaly | Improves symptoms; does not correct underlying disease |
Sarcoidosis | Hypersplenism or symptomatic splenomegaly | Improves symptoms and cytopenias; does not correct underlying disease |
Malignant Diseases | ||
Non-Hodgkin’s lymphoma | Cytopenias, symptomatic splenomegaly | Improved complete blood count values, relief of symptoms |
Hodgkin’s disease | Surgical staging in select cases | — |
Chronic lymphocytic leukemia | Hypersplenism or symptomatic splenomegaly | Improves symptoms and cytopenias (60–70% response rate) |
Hairy cell leukemia | Cytopenias and symptomatic splenomegaly | 40–70% response rate |
Acute myeloid leukemia | Intolerable symptomatic splenomegaly | Relief of abdominal pain and early satiety |
Chronic myeloid leukemia | Symptomatic splenomegaly | Relief of abdominal pain and early satiety |
Myelofibrosis (AMM) | Severe symptomatic splenomegaly | 76% clinical response at 1 year, high risk of hemorrhagic, thrombotic, and infectious complications (26%) |
Essential thrombocythemia and polycytemia vera | Only for advanced disease with severe symptomatic splenomegaly | Relief of abdominal pain and early satiety |
Idiopathic Thrombocytopenic Purpura (ITP)
ITP is the most common indication for elective splenectomy. It is an acquired disorder characterized by splenic production of immunoglobulin (Ig)G that induces splenic sequestration and the destruction of platelets. The hallmarks of this condition are low platelet levels, ecchymoses, purpura, petechiae, and abnormal bleeding.
The management of childhood ITP is different from that for adults, particularly with regard to the role of splenectomy. The condition is often self-limiting in children, with >70% of cases resolving spontaneously. Splenectomy for children is rarely indicated. It is reserved for the rare case of severe, symptomatic thrombocytopenia of >1 year duration that is refractory to medical management (including corticosteroids and intravenous infusion of immunoglobulin (IVIG)).
In adults, the thrombocytopenia associated with ITP is usually more severe and requires medical and surgical intervention. In general, splenectomy is indicated in adults with ITP if the patient does not improve after 8 weeks of corticosteroid therapy or if the thrombocytopenia recurs after the suspension of medical treatment. Intracranial hemorrhage is an indication for emergency IVIG and splenectomy.
The spleen is often not enlarged in ITP, making laparoscopic splenectomy an attractive option for these patients. Because the splenic tissue is the source of the disorder, a key component of the surgcial strategy is thorough inspec tion of the abdomen for ASs. In patients who have recurrent symptoms after splenectomy, a 99mTc-labeled RBC or 111In-labeled platelet scan can be employed to identify the location of the AS and facilitate resection, with removal of the remnant AS typically associated with ITP cure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree