1
Epidemiology
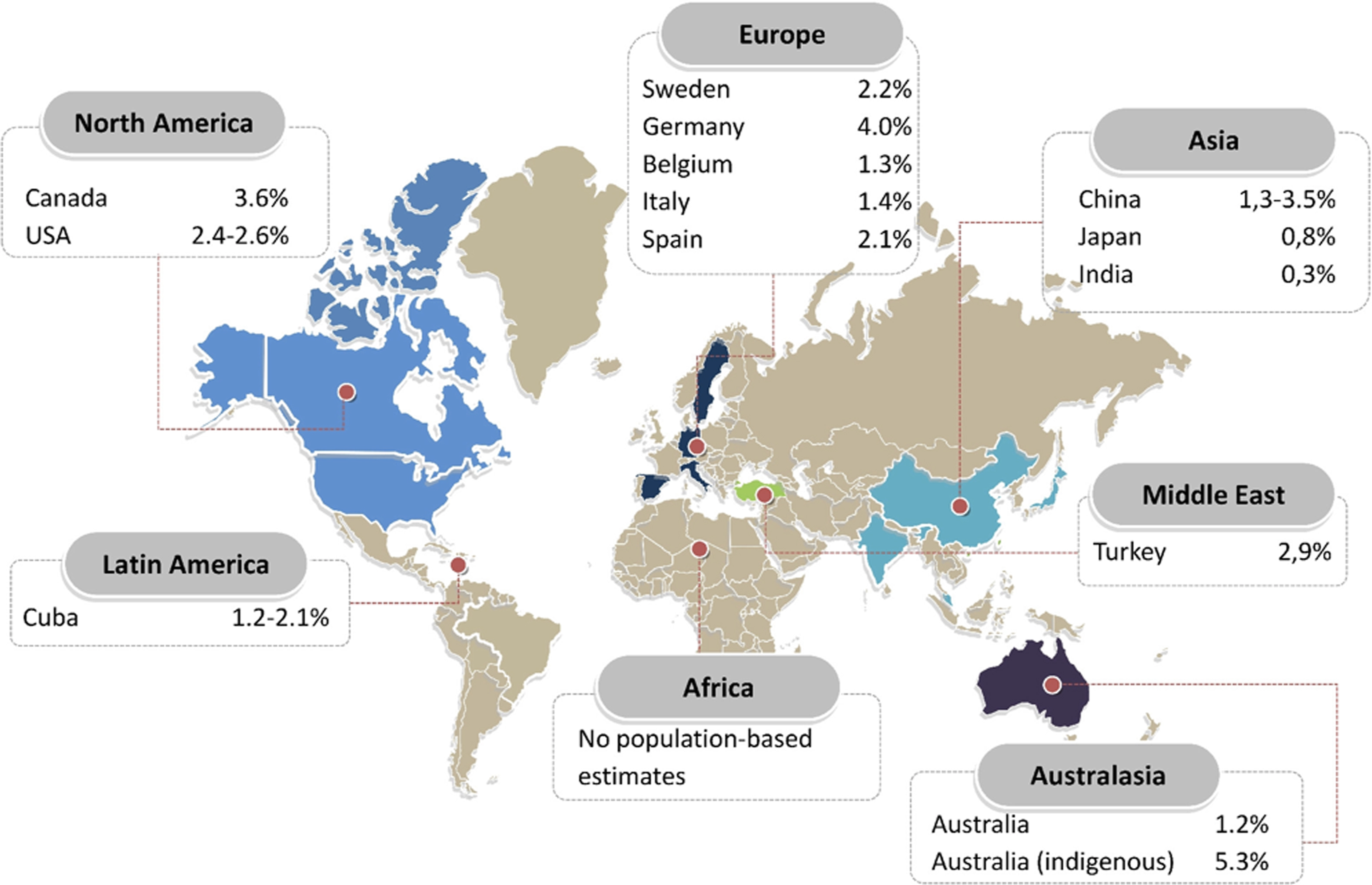
An estimated 64.3 million people are living with heart failure worldwide. In developed countries, the prevalence of known heart failure is generally estimated at 1%–2% of the general adult population ( Fig. 18.1 ) [ ]. A meta-analysis based on echocardiographic screening studies in the general population showed that the real prevalence of heart failure in developed countries is around 11.8% in those aged ≥65 years. This would account for a calculated prevalence in the general population of 4.2%; thus around twice as high as some of the reported prevalences based on registries containing only established cases. The difference between 4.2% and 2% illustrates that even a prognostically severe syndrome as heart failure may remain undetected in over half of the cases, especially heart failure with preserved ejection fraction (HFpEF). The absolute numbers of patients living with heart failure have been increasing, as a result of aging of the population, global population growth, and improved survival after diagnosis. Nevertheless, there is a concerning lack of epidemiological data from countries outside Europe and North America, especially from lower and middle-income countries, even though these are estimated to carry 80% of the cardiovascular disease burden [ ]. From scarce literature, heart failure prevalence in Asia seems to be rather similar compared to Western countries, ranging between 1% and 1.3%, although most studies rely on administrative data. A single population-based echocardiographic study investigating HFpEF in Northern China found a prevalence of 3.5% [ ]. The prevalence in Australia is 1%–2% based on national surveys, although echocardiographic and biomarker studies showed that the prevalence in Indigenous communities is 5.3%, despite a lower mean age [ ]. There are regional differences in heart failure phenotypes. For example, Asian HFpEF patients in the Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry are a decade younger than HFpEF patients in the Olmsted County cohort and have a relatively high comorbidity burden. Particularly, the diabetes burden is high in Asia, in spite of a much lower prevalence of overweight/obesity (21.6%–26.2% in Southeast Asia compared to 69.6% in the USA) [ ]. In Southeast Asia, a unique lean diabetic phenotype makes up for 20% of all heart failure cases and is associated with higher rates of all-cause mortality and hospitalization [ ].
The incidence of heart failure in European countries and the USA ranges widely from 1 to 9 cases per 1000 person-years and strongly depends, again, on the population studied and the diagnostic criteria used. In developed countries, incidence rates have stabilized between 1970 and 1990 and are now thought to be decreasing. In the population-based study by Conrad, a decline of 7% of all-type heart failure was noted between 2002 and 2014 from 3.6 to 3.3/1000 person-years [ ]. The decline was largely driven by a decline in patients aged 60–84 years. Worrisomely, the incidence remained stable or increased in younger patients (<55 years) and in the very old (>85 years), groups that have thus far received fairly little attention. Between 2000 and 2010, age- and sex-adjusted incidence declined substantially, from 3.2 to 2.2 cases per 1000 person-years. The decline was greater in women (43%) than in men (29%), and greater in heart failure with reduced ejection fraction (HFrEF) (45%) than in HFpEF (28%). The proportion of incident HFpEF cases in this cohort increased, simultaneously to an increase in the proportion of heart failure patients with hypertension, atrial fibrillation, and diabetes [ ]. Recent reliable European estimates of the incidence of heart failure come from the Prevention of Renal and Vascular End-stage Disease (PREVEND) study. Incidence rate was 3.7/1000 person-years in men and 2.4/1000 person-years in women; 34% of the cases were classified as HFpEF [ ]. Despite the decline in standardized incidence of heart failure, the total number of new cases of heart failure has increased by 12%, mainly as a result of a growing and aging population [ ].
Several population-based cohorts have examined common factors that predispose to heart failure, most notably coronary artery disease, hypertension, diabetes, obesity, and smoking. Except for smoking, the burden of risk factors in patients with heart failure is increasing over time, significantly so for diabetes, obesity, and hypertension [ ]. After decades of observing a steadily increasing incidence rate of diagnosed diabetes, US national surveillance data now show a sustained decline. However, this may simply be the result of depletion of the population at risk due to successful screening and detection in the previous years [ ]. Diabetes remains highly prevalent and is present in approximately 40% of HFrEF patients and 45% of HFpEF patients [ ]. Diabetes and obesity affect left ventricular function also in absence of coronary artery disease and hypertension. Importantly, the relationship between diabetes and heart failure seems to be bidirectional. In a Dutch study of 581 patients with type 2 diabetes aged >60 years, in whom the diagnosis of heart failure was not known, opportunistic screening revealed “new” heart failure in 27.7% of the participants (4.8% HFrEF and 22.9% HFpEF) [ ]. A recent study suggests that obesity and obesity-related cardiometabolic traits (including insulin resistance) are more strongly associated with the risk of incident HFpEF than HFrEF, especially in women [ ]. The Framingham Heart Study showed, after correcting for cardiovascular risk factors and coronary artery disease, that the frequency of heart failure was twice and five times higher in men and women with diabetes, respectively, than in age-matched nondiabetic subjects [ ]. Indeed, heart failure is one of the most common initial manifestations of cardiovascular disease in patients with type 2 diabetes mellitus. The risk of heart failure is also increased in patients with type 1 diabetes mellitus. These patients have been described as having a four-fold increase in the risk of being admitted to the hospital with heart failure, compared with population-based controls [ ]. In both type 1 [ ] and type 2 diabetes mellitus [ ], poor glycemic control has been described to be associated with an increased incidence of heart failure. Moreover, as shown in both observational and clinical studies, diabetes is associated with a poor subsequent prognosis in subjects with heart failure [ ]. In fact, in patients with type 2 diabetes mellitus, mortality is increased 10-fold in patients with versus those without heart failure [ ].
2
Definition and diagnosis
Heart failure is a clinical syndrome with current or prior symptoms (e.g., breathlessness, ankle swelling, and fatigue) and/or signs (e.g., elevated jugular venous pressure, pulmonary crackles, and peripheral edema) ( Table 18.1 ) caused by a structural and/or functional cardiac abnormality (as determined by left ventricular ejection fraction (LVEF) <50%, abnormal cardiac chamber enlargement, diastolic dysfunction (E/E′>15), moderate/severe ventricular hypertrophy or moderate/severe valvular obstructive or regurgitant lesion) and corroborated by at least one of the following: elevated natriuretic peptide levels (ambulatory: BNP ≥35 pg/mL, NT-proBNP ≥125 pg/mL; hospitalized/decompensated: BNP ≥100 pg/mL, NT-proBNP ≥300 pg/mL) and/or objective evidence of cardiogenic pulmonary or systemic congestion by diagnostic modalities such as imaging (e.g., by chest X-ray or elevated filling pressures by echocardiography) or hemodynamic measurement (e.g., right heart and pulmonary artery catheterization) at rest or with provocation (e.g., exercise) [ ].
| Symptoms of heart failure | |
| Typical | Breathlessness orthopnea a paroxysmal nocturnal dyspnea a reduced exercise tolerance a fatigue, tiredness b ankle swelling a inability to exercise a swelling of parts of the body other than ankles bendopnoea |
| Less typical | Nocturnal cough wheezing bloated feeling c postprandial satiety c loss of appetite decline in cognitive function, confusion(especially in the elderly) b depression dizziness, syncope b |
| Signs of heart failure | |
| More specific | Elevated jugular venous pressure a third heart sound a , summation gallop with third and fourth heartsounds, cardiomegaly, laterally displaced apical impulse, hepatojugular reflux, Cheyne–Stokes respiration in advanced heartfailure b |
| Less specific | Peripheral edema (ankle, sacral, scrotal) pulmonary rales, a unintentional weight gain (>2 kg/week) weight loss (in advanced heart failure) with muscle wasting and cachexia cardiac murmur, reduced air entry, and dullness to percussion at lung bases suggestive of pleural effusion, tachycardia, irregular pulse, tachypnoea, hepatomegaly/ascites, cold extremities, b oliguria narrow pulse pressure |
a Commonly used in clinical trials, registries, risk scoring and have been tested for sensitivity and specificity.
b Common in low perfusion, low cardiac output states.
c Can be typical in the setting of right heart failure or biventricular failure.
The current ACCF/AHA classification of heart failure includes two presymptomatic stages [ ], A (patients “at risk” due to cardiovascular comorbidities) and B (patients with structural heart disease or cardiomyopathies) that would not be covered under the above definition as having heart failure, which emphasizes symptoms and signs of heart failure; but heart failure syndrome is as a continuum of disease with certain stages, such as preheart failure.
According to universal definition and classification of heart failure proposed in a report by the most relevant cardiology societies, published in 2021 [ ], the latest published European Heart Failure guidelines [ ], updated a heart failure classification according to LVEF. The strongest argument to use LVEF to categorize heart failure is that LVEF defines a group known to respond to life-prolonging therapy from randomized controlled trials and LVEF also provides prognostic information. So the latest classification of heart failure is the following:
Reduced LVEF is defined as ≤40%, that is, those with a significant reduction in LV systolic function. This is designated as “HFrEF.”
Patients with an LVEF between 41% and 49% have mildly reduced LV systolic function, that is, “HFmrEF.” Retrospective analyses from randomized clinical trials in HFrEF or HFpEF that have included patients with ejection fractions in the 40%–50% range suggest that they may benefit from similar therapies to those with LVEF ≤40%. This supports the renaming of HFmrEF from “heart failure with mid-range ejection fraction” to “heart failure with mildly reduced ejection fraction.”
Those with symptoms and signs of heart failure, with evidence of structural and/or functional cardiac abnormalities and/or raised natriuretic peptides, and with an LVEF ≥50%, have heart failure with preserved ejection fraction (“HFpEF”).
The etiology of heart failure varies according to geography. In Western-type and developed countries, coronary artery disease and hypertension are predominant factors. With regard to ischemic etiology, HFmrEF resembles HFrEF, with a higher frequency of underlying coronary artery disease compared to those with HFpEF. The diagnosis of chronic heart failure is made more likely in patients with a history of myocardial infarction, arterial hypertension, coronary artery disease, diabetes mellitus, alcohol misuse, chronic kidney disease, cardiotoxic chemotherapy, and in those with a family history of cardiomyopathy or sudden death. The following diagnostic tests are recommended for the assessment of patients with suspected chronic heart failure [ ]:
Electrocardiogram (ECG). A normal ECG makes the diagnosis of heart failure unlikely. The ECG may reveal abnormalities such as atrial fibrillation, Q waves, left ventricular hypertrophy, and a widened QRS complex that increase the likelihood of a diagnosis of heart failure and also may guide therapy.
Measurements of natriuretic peptides are recommended, if available. A plasma concentration of BNP <35 pg/mL, NT-proBNP <125 pg/mL, or mid-regional proatrial natriuretic peptide (MR-proANP) <40 pmol/L make a diagnosis of heart failure unlikely.
Basic investigations such as serum urea and electrolytes, creatinine, full blood count, liver, and thyroid function tests are recommended to differentiate heart failure from other conditions, to provide prognostic information, and to guide potential therapy.
Echocardiography is recommended as the key investigation for the assessment of cardiac function. As well as the determination of the LVEF, echocardiography also provides information on other parameters such as chamber size, eccentric or concentric left ventricular hypertrophy, regional wall motion abnormalities, right ventricular function, pulmonary hypertension, valvular function, and markers of diastolic function. Exercise or pharmacological stress echocardiography may be used for the assessment of inducible ischemia those who are considered suitable for coronary revascularization. In patients with HFpEF, valve disease, or unexplained dyspnea, stress echocardiography might help clarify the diagnosis.
A chest X-ray is recommended to investigate other potential causes of breathlessness. It may also provide supportive evidence of heart failure.
Cardiac magnetic resonance imaging with late gadolinium enhancement, T1 mapping, and extracellular volume will identify myocardial fibrosis/scar, which are typically subendocardial for patients with ischemic heart disease in contrast to the mid-wall scar typical of dilated cardiomyopathy. In addition, cardiac magnetic resonance allows myocardial characterization.
Computed tomography coronary angiography may be considered in patients with a low to intermediate pretest probability of coronary artery disease, or those with equivocal noninvasive stress tests in order to exclude the diagnosis of coronary artery disease.
Single-photon emission computed tomography can also be used to assess myocardial ischemia and viability, myocardial inflammation, or infiltration. Scintigraphy with technetium (Tc)-labeled bisphosphonate has shown high sensitivity and specificity for imaging cardiac transthyretin amyloid.
Coronary angiography is recommended in patients with heart failure, who have angina pectoris or an “angina equivalent” despite pharmacological therapy, in order to establish the diagnosis of coronary artery disease and its severity. Coronary angiography may also be considered in patients with HFrEF who have an intermediate to high pretest probability of coronary artery disease and who are considered potentially suitable for coronary revascularization.
The diagnosis of HFpEF remains challenging. Several diagnostic criteria have been proposed by societies and in clinical trials. These criteria vary widely in their sensitivities and specificities for diagnosing HFpEF. To facilitate broad clinical application, the latest European Heart Failure Guidelines [ ] recommend a simplified pragmatic approach that distils the common major elements in prior diagnostic criteria and emphasizes the most frequently used variables widely available to clinicians. This simplified diagnostic approach starts with assessment of pretest probability. The diagnosis should include the following:
- 1.
Symptoms and signs of HF.
- 2.
An LVEF ≥50%.
- 3.
Objective evidence of cardiac structural and/or functional abnormalities consistent with the presence of left ventricular diastolic dysfunction/raised left ventricular filling pressures, including raised natriuretic peptides.
Acute heart failure refers to rapid or gradual onset of symptoms and/or signs of heart failure, severe enough for the patient to seek urgent medical attention, leading to an unplanned hospital admission or an emergency department visit. The diagnostic workup of acute heart failure starts at the time of the first medical contact and is continued throughout the initial patient pathway, aiming to identify the clinical presentation and to diagnose and manage any potentially reversible causes/precipitants/coexisting life-threatening conditions in a timely manner. In addition to clinical signs and symptoms, diagnostic workup includes ECG and echocardiography, if possible. Additional investigations, that is, chest X-ray and lung ultrasound may be used to confirm acute heart failure diagnosis, especially when natriuretic peptide testing is not available. Plasma natriuretic peptides levels should be measured if the diagnosis is uncertain and a point-of-care assay is available. Normal concentrations of natriuretic peptides make the diagnosis of acute heart failure unlikely. Cut-offs for acute heart failure are: BNP <100 pg/mL, NT-proBNP <300 pg/mL, and MR-proANP <120 pg/mL. Among other laboratory tests, troponin is useful for the detection of acute coronary syndrome although elevated levels are detected in the vast majority of patients with acute heart failure. Blood urea nitrogen or urea, serum creatinine, electrolytes, and antigen carbohydrate 125 may help tailor treatment. Detection of abnormal liver function identifies patients with a poor prognosis. Since both hypothyroidism and hyperthyroidism may precipitate acute heart failure, thyroid-stimulating hormone should be assessed in those with newly diagnosed acute heart failure.
3
Treatment of diabetes mellitus in patients with heart failure
Given the associations of cardiac dysfunction with glucose metabolism, cardiac energy reserve, and steatosis, metabolic interventions aiming to improve glucose metabolism might have beneficial impacts on cardiac function. Although intensive glycemic control does not seem to impact the outcomes of heart failure, the choice of the glucose-lowering drug may have a major impact on the development of heart failure and other cardiovascular outcomes [ ]. Conversely, antidiabetic drugs differ in their effects in patients with heart failure and preference must be given to drugs that are both safe and reduce heart failure-related events.
Metformin: Currently, the main clinical practice guidelines unanimously recommend the use of metformin as the initial therapy of choice in the vast majority of patients with type 2 diabetes mellitus. There are no data from randomized trials specifically designed to evaluate the effects of metformin on heart failure, either on incident heart failure or on safety and efficacy in patients with established heart failure. Until now, there are data that support the evidence that metformin is at least as safe as other hypoglycemic agents in patients with diabetes mellitus and heart failure, even in those cases with decreased ejection fraction [ ], based on observational studies. In a systematic review of observational studies, metformin was reported to be associated with a 20% lower adjusted death rate compared with other antihyperglycemic medications, mainly sulfonylureas [ ]. Moreover, data from observational studies showed that metformin leads to a reduction in all-cause mortality in patients with type 2 diabetes mellitus with congestive heart failure and appears to reduce the admissions from congestive heart failure in patients with congestive heart failure or with moderate chronic nephropathy [ ]. Furthermore, metformin has not been reported to be associated with an increased risk of lactic acidosis in patients with chronic heart failure or with mild chronic kidney disease [ ]. Hence, these data have prompted regulatory authorities to remove heart failure from the earlier list of product-label contraindications for metformin therapy [ ]. The Food and Drug Administration (FDA) withdrew congestive heart failure as a contraindication for the use of metformin in 2006. Also, in April 2016, the agency reviewed its warning regarding chronic nephropathy to restrict the use of metformin only in patients with severe disease (glomerular filtration rate <30 mL/min/1.73 m 2 ), thus allowing its administration in subjects with moderate chronic nephropathy (30–60 mL/min/1.73 m 2 ).
Sulfonylureas and insulin: As with metformin, clinical trial data are scarce concerning the impact on heart failure in subjects with established cardiovascular disease treated with either sulfonylureas or insulin. No difference in heart failure events was recorded in the UK Prospective Diabetes Study (UKPDS) comparing sulfonylureas or insulin treatment with dietary intervention in 3867 newly diagnosed patients with diabetes [ ]. Regarding sulfonylureas, population-based observational studies have provided discordant results [ , ]. Insulin is needed in patients with type 1 diabetes mellitus and to control hyperglycemia in some patients with type 2 diabetes mellitus, especially when beta-cell function is exhausted. It is a sodium-retaining hormone and concern has been raised that it may exacerbate fluid retention in patients with heart failure. However, in a randomized clinical trial that included patients with type 2 diabetes, impaired glucose tolerance, or impaired fasting glucose, insulin did not increase the risk of incident heart failure [ ]. Use of insulin was associated with poorer outcomes in retrospective analyses of randomized trials and administrative databases [ , ]. If insulin is needed in a patient with heart failure, the patient should be monitored for evidence of worsening of heart failure after treatment initiation. In summary, based on this limited evidence, the presence of heart failure is not a contraindication for the use of sulfonylurea therapy, but an alternative option of using another drug seems preferable.
Thiazolidinediones: The thiazolidinedione family of Peroxisome proliferator-activated receptor gamma (γ) (PPARγ) agonists initially provided a promising therapeutic option in type 2 diabetes mellitus owing to antidiabetic efficacy combined with pleiotropic beneficial cardiovascular effects. However, the utility of thiazolidinediones in type 2 diabetes mellitus has declined in the past decade, largely due to concomitant adverse effects of fluid retention and edema formation attributed to salt-retaining effects of proliferator-activated receptor γ activation on the nephron. The two major cardiovascular outcome trials with these drugs, Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycemia and Diabetes (RECORD) and PROspective pioglitAzone Clinical Trial In macroVascular Events (PROactive), identified a substantial increase in risk of heart failure, although heart failure was not a prespecified adjudicated endpoint in PROACTIVE [ ]. In this latter study, a significant reduction in the secondary outcome (cardiovascular death, and nonfatal myocardial infarction or stroke) occurred (HR 0.84), although there was no significant reduction in the primary end-point, that included also revascularization procedures. More recently, treatment with pioglitazone showed superiority for major cardiovascular events in a population of 3895 patients with recent stroke and insulin resistance (although not type 2 diabetes mellitus) randomized to pioglitazone versus placebo in the Insulin Resistance Intervention After Stroke trial, with no significant increase in HF outcomes [ ]. However, thiazolidinediones have a fluid retention potential, leading to increased heart failure events. Already in 2003, the American Diabetes Association and the American Heart Association recommended that thiazolidinediones should be used with caution in subjects with class I-II of the New York Heart Association, while they should be avoided in subjects with class III and IV [ ]. The European Society of Cardiology stated in their 2021 guidelines that thiazolidinediones are contraindicated in patients with heart failure [ ].
Dipeptidyl peptidase IV (DPP-4) Inhibitors: Studies in different experimental models suggest that these drugs may have renoprotective and cardioprotective effects. However, conflicting findings have been described in other preclinical animal models and also in clinical studies. The cardiovascular effects of DPP-4 inhibitors remain controversial. While these drugs did not reduce or increase the risk of primary, prespecified composite cardiovascular outcomes, one DPP-4 inhibitor (sitagliptin) demonstrated no effect on heart failure [ ]. Another (saxagliptin) increased the risk of hospitalization for heart failure in the overall population [ ], and a third (alogliptin) demonstrated inconsistent effects on heart failure hospitalization across subgroups of patients [ ]. No difference over placebo for heart failure events was found with linagliptin [ ]. Vidagliptin was associated with an increase in left ventricular volumes and a numerically greater number of deaths and cardiovascular events in a small trial in patients with diabetes and heart failure [ ]. The mechanisms related to the increased heart failure risk with saxagliptin and the evident heterogeneity with regard to heart failure effects within the DPP4 inhibitor class requires further elucidation. These drugs are therefore not recommended to reduce cardiovascular events in diabetic patients with heart failure [ ].
GLP-1 receptor agonists (GLP-1RA): No increased risk of hospitalization for heart failure has been reported with GLP-1RAs in meta-analyses of phase-II/III trials (exenatide, albiglutide, dulaglutide, liraglutide) [ ], demonstrating the safety of this pharmacological class, and these findings have been confirmed in three large prospective cardiovascular outcome trials (Evaluation of Lixisenatide in Acute Coronary Syndrome (ELIXA) with lixisenatide, Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Results (LEADER) with liraglutide and the Trial to Evaluate Cardiovascular and other long-term outcomes with Semaglutide in Subjects with Type 2 Diabetes (SUSTAIN-6) with semaglutide). In particular, the LEADER trial reported a trend toward a reduction in HF hospitalization (13%, P 1⁄4 0.14), together with a significant reduction in cardiovascular and all-cause mortality in patients with type 2 diabetes mellitus at risk for cardiovascular disease [ ]. These results are reassuring in the face of the somewhat negative results of the Functional Impact of GLP-1 for Heart Failure Treatment (FIGHT) trial, which evaluated the effects of liraglutide in patients with advanced heart failure and low LVEF, such that further studies and caution are now required when using this agent to treat these patients in clinical practice. In LIVE trial, liraglutide significantly decreased hemoglobin A1c and increased 6-min walking test and increased heart rate and serious cardiac adverse events, and there were no statistical differences in LVEF, NT-proBNP, left ventricular end-systolic volume index, and left ventricular end-diastolic volume index [ ]. GLP-1 receptor agonists are therefore not recommended for the prevention of heart failure events.
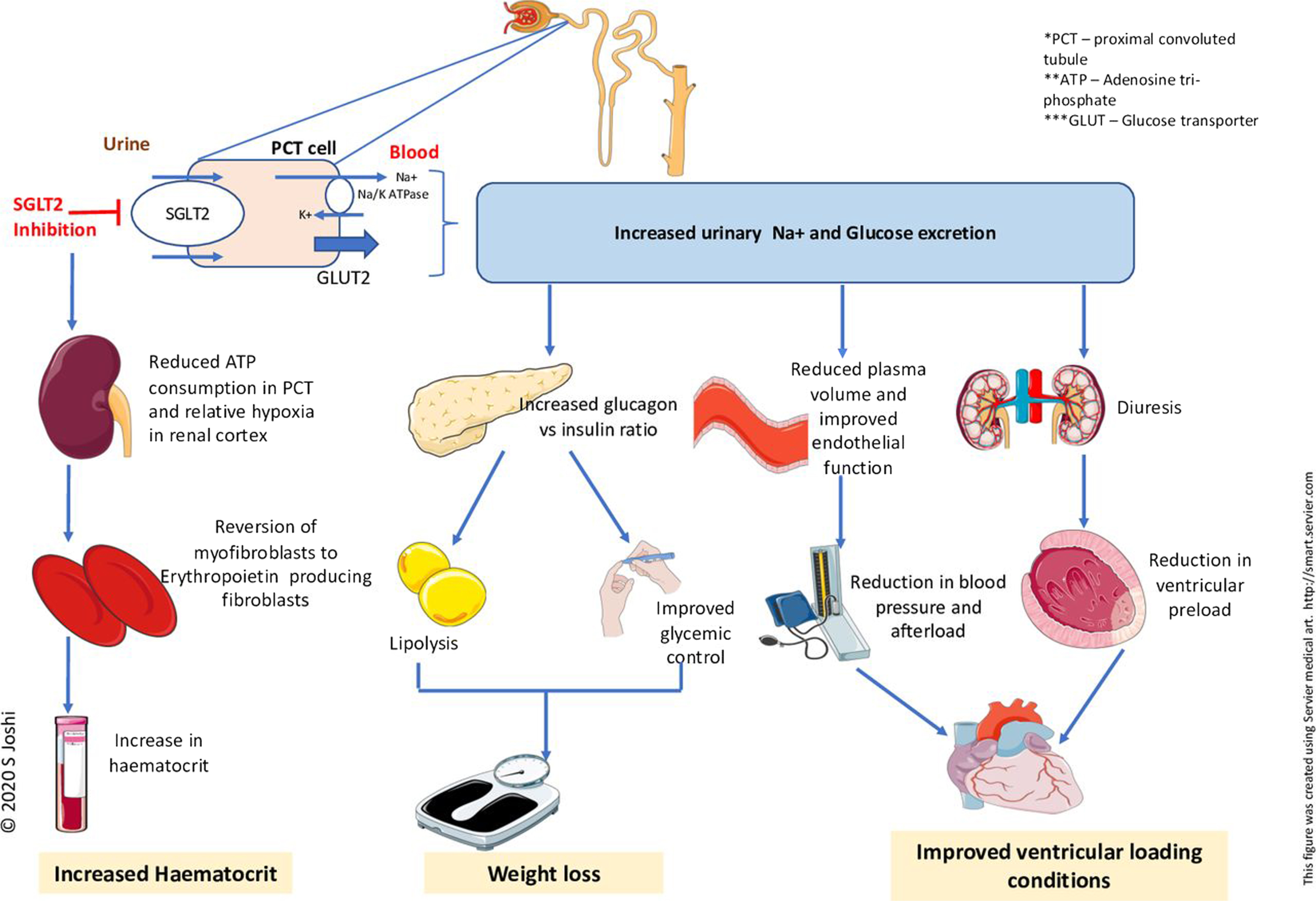
Sodium-glucose transporter type 2 (SGLT2) inhibitors: The SGLT2 inhibitors canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, and sotagliflozin were studied in patients with established cardiovascular disease in the EMPA-REG OUTCOME and VERTIS-CV trials, with established cardiovascular disease or cardiovascular risk factors in the CANVAS and DECLARE-TIMI 58 trials, and with chronic kidney disease and cardiovascular risk in the SCORED trial, respectively [ ]. A small proportion of patients had a history of heart failure in these trials. Empagliflozin and canagliflozin reduced the primary composite endpoint of major cardiovascular adverse events, including cardiovascular death or nonfatal myocardial infarction or nonfatal stroke, and heart failure hospitalizations in EMPA-REG OUTCOME and CANVAS, respectively [ , ]. Empagliflozin also reduced all-cause death or cardiovascular death alone [ ]. The effects on the primary endpoint were driven by the reduction in heart failure-related events in EMPA-REG OUTCOME and CANVAS. In DECLARE-TIMI 58, dapagliflozin did not reduce major cardiovascular events but reduced the co-primary efficacy endpoint of cardiovascular death or heart failure hospitalization and heart failure hospitalization alone [ ]. In VERTIS-CV, neither the primary major cardiovascular event endpoint nor the key secondary outcome of cardiovascular death or heart failure hospitalization were reduced significantly by ertugliflozin, although there was a statistically significant reduction in heart failure hospitalization and repeated hospitalizations [ ]. In SCORED, sotagliflozin reduced cardiovascular deaths and heart failure hospitalizations [ ]. In a meta-analysis of these trials and one further trial in patients with chronic kidney disease (CREDENCE), overall SGLT2 inhibitors reduced heart failure and cardiovascular hospitalization by 22% [ ]. SGLT2 inhibitors were well tolerated, although they may cause genital fungal skin infections and, rarely, diabetic ketoacidosis. Trial results with dapagliflozin and empagliflozin in patients with HFrEF, with or without diabetes, and with the SGLT1/2 inhibitor sotagliflozin in patients with type 2 diabetes stabilized after hospitalization for acute heart failure or within 3 days after discharge, further support the administration of these agents [ ]. EMPA-REG OUTCOME, CANVAS, DECLARE-TIMI 58, and VERTIS-CV also showed a reduction in worsening renal function, end-stage renal disease or death from renal causes, with SGLT2 inhibitors. Based on these results, the SGLT2 inhibitors canagliflozin, dapagliflozin, empagliflozin, ertugliflozin, or sotagliflozin are recommended to prevent heart failure and cardiovascular death and worsening kidney function in patients with type 2 diabetes and cardiovascular disease and/or cardiovascular risk factors, or chronic kidney disease. Dapagliflozin and empagliflozin are also indicated for the treatment of patients with type 2 diabetes and HFrEF and sotagliflozin was shown to reduce cardiovascular deaths and heart failure re-hospitalizations in patients recently hospitalized for heart failure [ , ].
4
Medical therapy for heart failure in patients with diabetes mellitus
Current guidelines from the European [ ] as well as the American [ ] cardiology societies do not recommend specific heart failure treatment approaches in patients with diabetes. There is no distinction between patients with or without diabetes to decide heart failure treatment. So, in this section, our approach takes into consideration treatments according to LVEF and is divided into: (1) treatment in patients with HFrEF, (2) treatment in patients with HFmrEF, and (3) treatment in patients with HFpEF. Likewise, treatment in patients with HFrEF is divided into drugs recommended in all patients with HFrEF and other drugs recommended or to be considered in selected patients with HFrEF.
4.1
Pharmacological treatment of heart failure with reduced ejection fraction
There are three major goals of treatment for patients with HFrEF: reduction in mortality, prevention of recurrent hospitalizations due to worsening heart failure, and improvement in clinical status, functional capacity, and quality of life. Pharmacotherapy is the cornerstone of treatment for HFrEF and should be implemented before considering device therapy, and alongside nonpharmacological interventions, according to the latest european clinical guidelines [ ].
4.1.1
Drugs recommended in all patients with HFrEF
Angiotensin converting enzyme inhibitors (ACE-Is) were the first class of drugs shown to reduce mortality and morbidity in patients with HFrEF [ ]. They have also been shown to improve symptoms [ ]. They are recommended in all patients unless contraindicated or not tolerated. They should be uptitrated to the maximum tolerated recommended doses.
Beta-blockers have been shown to reduce mortality and morbidity in patients with HFrEF, in addition to treatment with an ACE-I and diuretic [ ]. They also improve symptoms [ ]. There is consensus that ACE-I and beta-blockers can be commenced together as soon as the diagnosis of symptomatic HFrEF is established. There is no evidence favoring the initiation of a beta-blocker before an ACE-I and vice versa. Beta-blockers should be initiated in clinically stable, euvolemic patients, at a low dose, and gradually uptitrated to the maximum tolerated dose. In patients admitted with acute heart failure, beta-blockers should be cautiously initiated in hospital, once the patient is hemodynamically stabilized.
Mineralocorticoid receptor antagonists (MRAs) are recommended, in addition to an ACE-I and a beta-blocker, in all patients with HFrEF to reduce mortality and the risk of heart failure hospitalization [ , ]. They also improve symptoms. Eplerenone is more specific for aldosterone blockade and, therefore, causes less gynecomastia. Caution should be exercised when MRAs are used in patients with impaired renal function and in those with serum potassium concentrations >5.0 mmol/L.
Angiotensin receptor-neprilysin inhibitor (ARNI) . In the PARADIGM-HF trial, sacubitril/valsartan, an ARNI, was shown to be superior to enalapril in reducing hospitalizations for worsening heart failure, cardiovascular mortality, and all-cause mortality in patients with ambulatory HFrEF with LVEF ≤40%. Patients in the trial had elevated plasma natriuretic peptides concentrations, an eGFR ≥30 mL/min/1.73 m 2 and were able to tolerate enalapril and then sacubitril/valsartan during the run-in period [ ]. Additional benefits of sacubitril/valsartan included an improvement in symptoms and quality of life [ , ], a reduction in the incidence of diabetes requiring insulin treatment [ ], and a reduction in the decline in eGFR [ ], as well as a reduced rate of hyperkalemia [ ]. Additionally, the use of sacubitril/valsartan may allow a reduction in loop diuretic requirement [ ]. Symptomatic hypotension was reported more commonly in patients treated with sacubitril/valsartan as compared to enalapril, but despite developing hypotension, these patients also gained clinical benefits from sacubitril/valsartan therapy [ , ]. Therefore, it is recommended that an ACE-I or ARB is replaced by sacubitril/valsartan in ambulatory patients with HFrEF, who remain symptomatic despite optimal treatment outlined above. Two studies have examined the use of ARNI in hospitalized patients, some of whom had not been previously treated with ACE-I. Initiation in this setting appears safe and reduces subsequent cardiovascular death or heart failure hospitalizations by 42% compared to enalapril [ ]. As such, initiation of sacubitril/valsartan in ACE-I naive (i.e., de novo) patients with HFrEF may be considered (class of recommendation IIb, level of evidence B). Patients being commenced on sacubitril/valsartan should have an adequate blood pressure and an eGFR ≥30 mL/min/1.73 m 2 . A washout period of at least 36 h after ACE-I therapy is required in order to minimize the risk of angioedema.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors. The DAPA-HF trial investigated the long-term effects of dapagliflozin compared to placebo in addition to optimal medical therapy, on morbidity and mortality in patients with ambulatory HFrEF. Patients participated in the trial if they were in NYHA class II–IV and had an LVEF ≤40% despite optimal medical therapy. Patients were also required to have an elevated plasma NT-proBNP and an eGFR ≥30 mL/min/1.73 m 2 [ ]. Therapy with dapagliflozin resulted in a 26% reduction in the primary endpoint: a composite of worsening heart failure (hospitalization or an urgent visit resulting in i.v. therapy for heart failure) or cardiovascular death. Both of these components were significantly reduced. Moreover, dapagliflozin reduced all-cause mortality [ ], alleviated heart failure symptoms, improved physical function, and quality of life in patients with symptomatic HFrEF [ ]. Benefits were seen early after the initiation of dapagliflozin, and the absolute risk reduction was large. Survival benefits were seen to the same extent in patients with HFrEF with and without diabetes and across the whole spectrum of HbA1c values. Subsequently, the EMPEROR-reduced trial found that empagliflozin reduced the combined primary endpoint of cardiovascular death or heart failure hospitalization by 25% in patients with NYHA class II–IV symptoms, and an LVEF ≤40% despite optimal medical therapy [ ]. This trial included patients with an eGFR >20 mL/min/1.73 m 2 , and there was also a reduction in the decline in eGFR in individuals receiving empagliflozin. It was also associated with an improvement in quality of life [ ]. Although there was not a significant reduction in cardiovascular mortality in the EMPEROR-reduced trial, a recent meta-analysis of the DAPA-HF and EMPEROR-reduced trials found no heterogeneity in cardiovascular mortality [ ]. Therefore, dapagliflozin or empagliflozin is recommended, in addition to optimal medical therapy with an ACE-I/ARNI, a beta-blocker, and an MRA, for patients with HFrEF regardless of diabetes status. The diuretic/natriuretic properties of SGLT2 inhibitors may offer additional benefits in reducing congestion and may allow a reduction in loop diuretic requirement [ ]. The combined SGLT-1 and 2 inhibitor, sotagliflozin, has also been studied in patients with diabetes who were hospitalized with heart failure. The drug reduced cardiovascular death and hospitalization for heart failure [ ]. Therapy with SGLT2 inhibitors may increase the risk of recurrent genital fungal infections. A small reduction in eGFR following initiation is expected and is reversible and should not lead to premature discontinuation of the drug. Fig. 18.2 shows conventional mechanisms of action of SGLT2 inhibitors.