1
Introduction
Among the most remarkable advances in ophthalmology over the last decades has been the discovery of vascular endothelial growth factor (VEGF) inhibitors for the treatment of different retinal vascular diseases, including diabetic retinopathy (DR). Such a revolutionary therapeutic approach is of unquestionable importance in the shift of the management landscape for DR, which is still rapidly evolving. Novel therapeutic agents are in the development pipeline and could prove promising for diabetic eye disease treatment in a short span of time. A major facilitator for this has been the convergence of novel retinal imaging modalities and the noteworthy developments in proteomics that has fostered the understanding of microscopic and molecular ophthalmic features associated with DR in the postgenomic era. That, coupled with the advances in bioengineering technology has spun off parallel avenues of research to address the inherent limitations of anti-VEGF therapy. In particular, the burdensome urgency for injections and the related follow-up visits represent the major reasons for nonadherence to treatment that ought to be considered of utmost importance to prevent irreversible vision loss. Furthermore, the response to anti-VEGF observed in real-world outcomes is often inadequate and results in suboptimal vision gains, speaking in favor of the existence of other determinants beyond VEGF that are not being targeted so far. Therefore, it entails a continued endeavor in research toward two main directions: on one hand, the development of new pharmacotherapeutics that target different molecular pathways in DR to address the multifactorial pathophysiology, including anti-inflammatory corticosteroid-related and non-anti-inflammatory corticosteroid-related molecules; on the other hand, the development of longer-lasting therapies to lessen the treatment burden, such as longer-acting formulations of anti-VEGF drugs, sustained-delivery implants, depot formulations, combination strategies, and gene therapies.
Hence, therapeutic improvements in the treatment of DR are expected over the next few years aiming at providing functional and morphologic outcomes improvement and sustained efficacy. Here, we present the state of the art of novel therapeutics for DR that are being tested. Also the current treatment approaches will be briefly addressed in order to better understand the potential of novel therapeutic targets in the management of this potentially blinding condition.
2
Current therapeutic strategies
Laser photocoagulation and vitrectomy introduced in the 1960s were among the first treatments introduced for the complications of DR—diabetic macular edema (DME) and proliferative diabetic retinopathy (PDR)—are still playing an important role today.
Photocoagulation treatment of the retina in patients affected by DR became the gold standard in the following decades since two major studies were published. The Diabetic Retinopathy Study (DRS) showed that panretinal photocoagulation (PRP) could reduce the severe vision loss (visual acuity ≤5/200) by 50% and the progression to more serious stages of proliferative retinopathy. The Early Treatment Diabetic Retinopathy Study (ETDRS) demonstrated that immediate focal photocoagulation was effective in reducing the risk of moderate vision loss caused by DME by at least 50% [ ]. Significant adverse effects of PRP and scatter photocoagulation were documented including and not limited to visual acuity loss and peripheral visual field constriction; however, the positive outcomes of the treatment outweighed these harmful risks in eyes either with high-risk PDR or DME that threatened the center of the macula. In addition, laser photocoagulation, although providing stabilization of visual acuity, failed to achieve clinically significant visual improvement, and therefore, its primary goal was to slow the rate of further vision loss [ ].
The advent of anti-VEGF therapy opened new therapeutic horizons in ophthalmology. Compared to conventional laser therapy, the intravitreal administration of anti-VEGF treatment had less ocular harmful effects and could achieve functionally meaningful (>5 ETDRS letters) visual improvement, demonstrating to be clinically effective in both DME and, more recently in PDR. By shifting the target from sight maintenance alone to sight restoration, the introduction of antiangiogenic therapy triggered a radical change in the treatment of DR-related microvascular complications [ , ]. Since 1994, when the central role of VEGF in the pathogenesis of DR/DME was first elucidated, anti-VEGF agents have increasingly become the first-line therapy for DME. In physiological conditions, VEGF acts on receptors VEGFR1 and VEGFR2 playing a key role in angiogenesis and ensuring the survival of retinal cells; in the hypoxic retinal environment of DR, VEGF production is upregulated, playing a key role in the pathogenesis of both DME and PDR [ , ]. Anti-VEGF agents that have been tested in clinical trials in the treatment of DR, especially DME, include pegaptanib (Macugen, Valeant, Madison, NJ, USA) [ ], bevacizumab (Avastin, Genentech, South San Francisco, CA, USA) [ ], ranibizumab (Lucentis, Genentech, South San Francisco, CA, USA) [ ], aflibercept (Eylea, Regeneron, Tarrytown, NY) [ ], and ziv-aflibercept (Zaltrap, Regeneron, Tarrytown, NY) [ ]. Regarding their clinical deployment, pegaptanib is no longer used due to its limited efficacy, bevacizumab and ziv-aflibercept—both approved for the treatment of metastatic solid tumors—are deployed as intravitreal off-label treatments, whereas ranibizumab and aflibercept are currently the only available on-label VEGF inhibitors used for DME.
Among these antiangiogenic agents, ranibizumab has been the most thoroughly tested along three major clinical lines: Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol I trial, RISE/RIDE studies, and RESTORE/RESOLVE trials. The DRCR.net Protocol I trial was the first to demonstrate with a level I evidence that Ranibizumab with prompt or deferred macular laser photocoagulation was superior than laser therapy considered as the standard of care in the treatment of center-involving DME [ , ]. The results of RISE/RIDE studies led to the US FDA approval of ranibizumab 0.3 mgfor the treatment of DME. These trials compared the efficacy of ranibizumab at 0.3 and 0.5 mg doses against sham injections, showing that a significant proportion of eyes improved by at least 15 letters—the primary functional endpoint—in RISE (45%, 39%, 18%) and RIDE (34%, 46%, 12%) at 24 months, and in mean visual acuity in the ranibizumab-treated groups in RISE (+13, +12, +3) and RIDE (+12, +11, +0.5) [ ]. Finally, the RESTORE/RESOLVE trials corroborated these findings, demonstrating that patients with DME gained greater best-corrected visual acuity (BCVA) with intravitreal ranibizumab than laser monotherapy and conducted to the EMA approval of 0.5 mg Ranibizumab for DME in Europe [ , ].
Also, intravitreal aflibercept was associated with better improvements in BCVA compared to standard laser photocoagulation in DME patients in VISTA and VIVID trials, resulting in the US FDA approval [ ]. Moreover, the DRCR.net, comparing the three commonly used anti-VEGF agents for DME at 2 years in Protocol T, showed that all of them could improve BCVA similarly in patients with BCVA >69 letters ETDRS with low rates of side effects, whereas in patients with lower baseline BCVA aflibercept and ranibizumab (0.3 mg) showed to be more effective than bevacizumab (with faster effect of aflibercept) in gaining vision [ ]. Unlike DME, the mainstay treatment for PDR remains PRP, though anti-VEGF therapy has been recently demonstrated to be noninferior in visual acuity change in Protocol S and CLARITY studies [ , ].
The aforementioned clinical studies have shaped the current approaches to the therapy of DR. However, anti-VEGF treatment requires frequent intravitreal injections regimens, leading to financial burden and poor patient compliance. Moreover, a significant percentage of cases lack response to antiangiogenic therapy and may still require laser photocoagulation as an adjuvant treatment. The identification of the role of inflammation in DR pathogenesis has led the corticosteroid to take an active role in the management of DME. Intravitreal injections of corticosteroids have been demonstrated to be clinically effective in DME, improving visual acuity and reducing excess foveal thickness in both nonresponders to laser and/or anti-VEGF therapy and also in treatment-naïve eyes [ ]. The lack of response is presumed to be driven by proinflammatory cytokines and chemokines, that are significantly upregulated in DR. Corticosteroids are potent anti-inflammatory drugs that target a broad range of pathogenetic mediators of DME [ , ]. Therefore, interest in corticosteroid use in the management of patients with DME has developed. Results from MEAD and FAME Phase III clinical trials for dexamethasone and fluocinolone acetonide, respectively, led to the FDA and EMA approval of intravitreal dexamethasone implant (Ozurdex, Allergan, Irvine, CA) [ ], fluocinolone acetonide insert (Iluvien, Alimera, Alpharetta, GA) [ ] for DME. Also worth mentioning is the off-label use of intravitreal triamcinolone acetonide [ ]. All intravitreal injections of steroids are associated with a longer mean duration of action compared to anti-VEGF agents, but with a less favorable safety profile, with a higher incidence of elevated intraocular pressure, and cataract. For this reason, corticosteroid use is regarded mostly as a second-line therapy by the scientific community.
Of note is the recent use of novel laser strategies for the treatment of DME. In particular, subthreshold micropulse laser (SMPL) showed to have superior functional gain and comparable results in reducing central macular thickness as standard ETDRS laser [ , ]. Moreover, SMPL showed to be a safe and effective treatment that does not destroy the retina but normalizes/restores the normal retina [ ]. However, the lack of standardization has limited its use in clinical practice.
As the last resort, pars plana vitrectomy has been usually performed for eyes that fail other treatments, for advanced complications of PDR, including vitreous hemorrhage and traction retinal detachment, and also for DME with vitreomacular traction [ ]. However, mixed visual outcomes have been reported.
The currently available treatments, which include intravitreal anti-VEGF agents and steroids, laser photocoagulation, and vitrectomy, reflect the complex pathogenesis of DR. Even though clinical benefits are reported, none of these options is yet to entirely mitigate the progression or reverse retinal damage in DR. In addition, they carry potentially serious sight-threatening adverse effects, and importantly, they do not focus on the prevention or the very early-stage disease. Therefore, several alternative molecular mechanisms underlying the pathophysiology of DR have been considered as potential therapeutic targets for the condition. Strong emphasis has been placed on optimization of current treatment regimens, strategies for combination therapy, and development of novel pharmacological drugs to reduce the burden of DR aiming to major impact on diabetic patient care as well as cost-effectiveness of management.
3
Novel therapeutic strategies
This section discusses the current status of novel treatment strategies under investigation for DME/DR. The therapeutic agents discussed in this chapter will be subdivided into several categories according to the mechanism of action. Particular focus is paid to carefully planned, randomized Phase III and II clinical trials with major availability of data. Drugs that have not been subjected to rigorous clinical trials or other molecules that are in early preclinical development have been omitted.
3.1
Blocking more than the anti-VEGF pathway
3.1.1
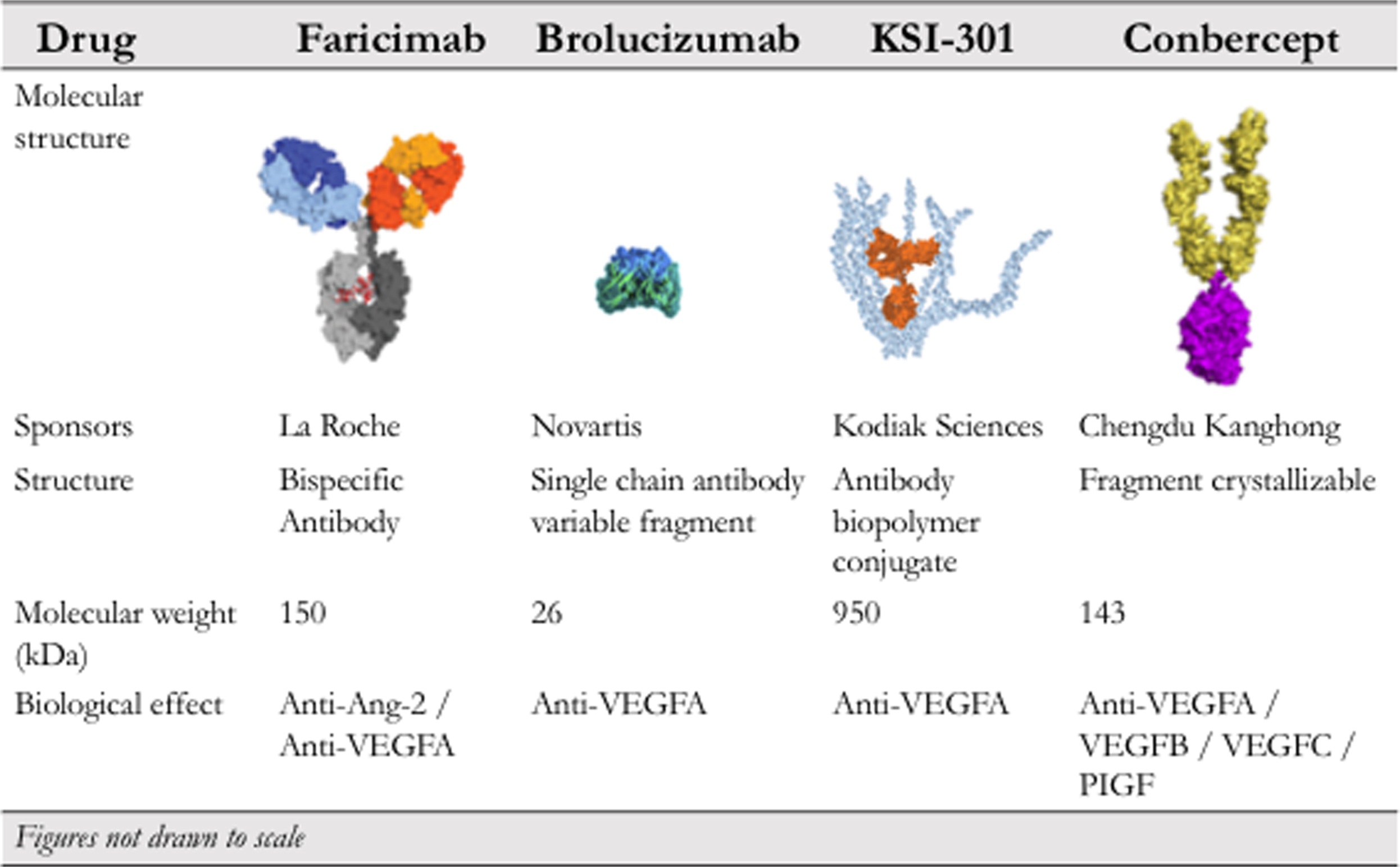
Faricimab
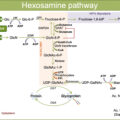
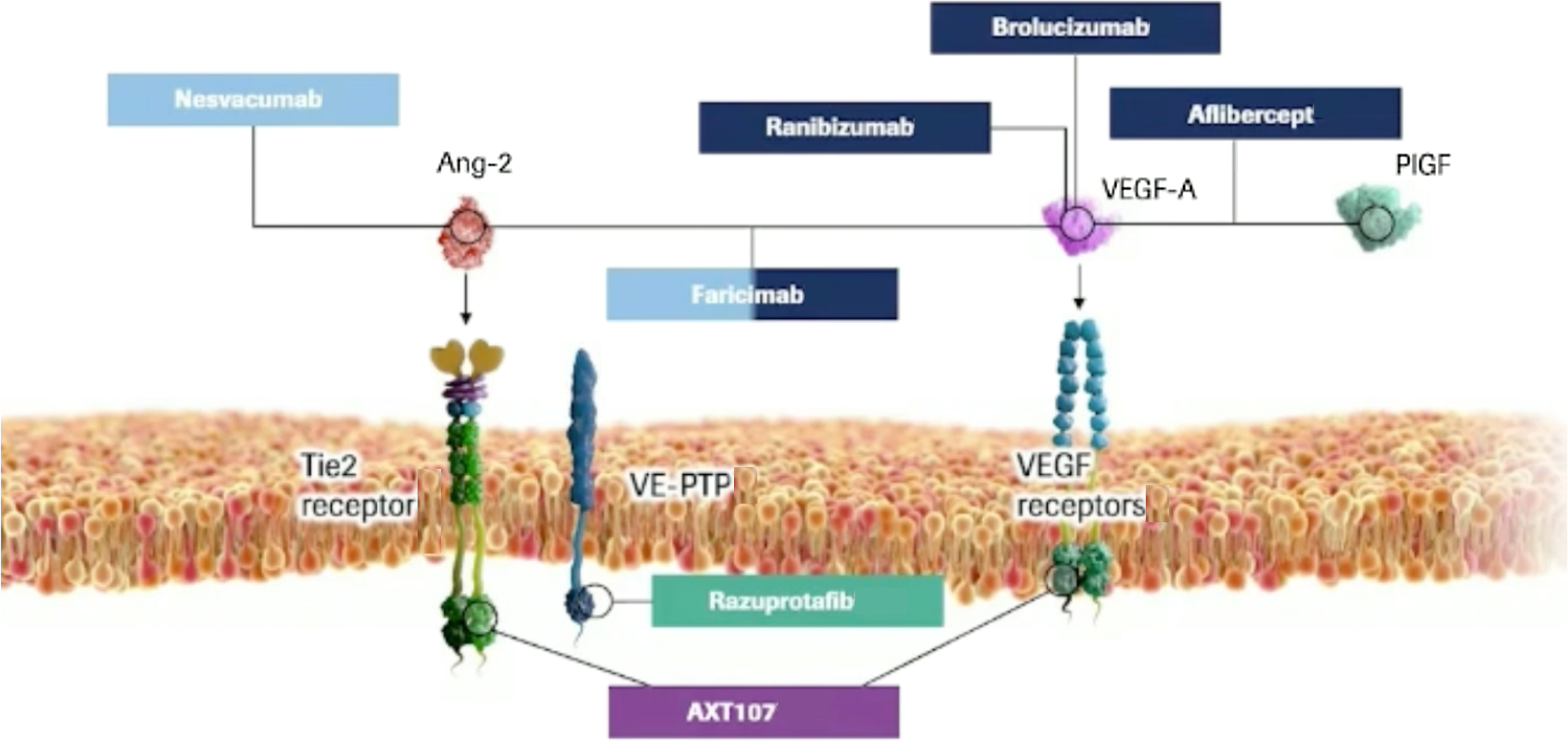
Faricimab (Hoffmann-La Roche, Basel, Switzerland), formerly known as RG7716, is a first-in-class bispecific humanized monoclonal IgG 1 antibody, designed to target two distinct pathways implicated in DR through two specific ligand-binding arms, namely angiopoietin-2 (Ang-2) and VEGF-A. VEGF-A isoforms play a key role in ocular angiogenesis, stimulate vascular dilation and permeability, and regulate the proliferation, migration, and survival of vascular endothelial cells.
The angiopoietin/Tie pathway is one of the most important contributors to angiogenesis and vascular permeability and includes the following growth factors: Ang-1, Ang-2, Ang-4, receptor tyrosine kinases Tie1 and Tie2, and vascular endothelial protein tyrosine phosphatase (VE-PTP). Of note, both Ang-2 and VEGF concentration in the vitreous of DR patients have been found abnormally elevated compared to healthy eyes, leading to a pathological state of vascular destabilization [ , ]. By synergically inhibiting both receptors, Faricimab may promote vascular stability, reduce inflammation and vascular leakage, and inhibit neovascularization and thus improve outcomes over anti–VEGF monotherapy alone ( Fig. 19.1 ).
The Phase II clinical trial (BOULEVARD) evaluated the efficacy and tolerability of faricimab compared with ranibizumab in patients with DME, previously naïve to anti-VEGF treatment. The primary endpoint was mean change from baseline in BCVA at Week 24. Faricimab demonstrated statistically significant BCVA gains over ranibizumab at Week 24 (13.9 letters versus 10.3 letters) [ ]. Based on the results of the BOULEVARD trial, two Phase III trials were initiated for DME: YOSEMITE ( NCT03622580 ) and RHINE ( NCT03622593 ) are two 100-week, randomized, double-masked, Phase III studies that enrolled a total of 1891 adult patients with center-involving DME and BCVA between 20/40–20/320, across 353 study sites worldwide to evaluate the efficacy and safety of faricimab versus aflibercept. Patients were equally randomized to three treatment arms: faricimab 6.0 mg administered at fixed 8-weeks (Q8W) intervals, faricimab 6.0 mg administered at a personalized treatment intervals (PTI), which means extended and tailored dosing intervals of up to 4 months based on the treat and extend concept, or aflibercept 2.0 mg Q8W. The primary endpoint is the mean change in best-corrected visual acuity (BCVA) score from baseline at year 1, averaged over week 48, 52, and 56. At year 1, YOSEMITE and RHINE trials consistently showed that faricimab is not inferior to aflibercept in visual acuity gains, with improved anatomic outcomes and potential for extended dosing, meeting the primary endpoint. In YOSEMITE, the average BCVA gains at year 1 from baseline were +11.6, +10.7, +10.9 ETDRS letters in the faricimab PTI, faricimab Q8W, and aflibercept arms, respectively; in RHINE, the results were +10.8 and +11.8, +10.3 letters, respectively. Of note, across the two studies more than 70% of faricimab PTI patients could extend the treatment to every 4 months in the first year, demonstrating greater central subfield thickness reductions compared to aflibercept Q8W arm. For what concerns safety outcomes, the drug was well tolerated, with reported intraocular inflammation events on average in 1.3% and 0.6% in faricimab and aflibercept arms, respectively, and without any case of retinal vasculitis. Data from the two trials at year 2 and the long-term extension RHONE X trial will further elucidate the long-term efficacy, safety, and durability of faricimab for the treatment of DME patients. Notably, faricimab is the first intravitreal injectable eye drug (excluding slow-release systems) that has achieved a 4-month interval between treatments in Phase III for DME.
3.1.2
Other molecules targeting the angiopoietin/Tie pathway
Besides faricimab, three other molecules targeting the angiopoietin/Tie pathway in DME have been evaluated in Phase II trials so far (Fig. 22.1).
AXT107 is a synthetic peptide derived from collagen IV with dual mechanism of action involving inhibition of VEGF receptor-2 (VEGFR2) thus VEGF-A and VEGF-C and activation of Tie-2 receptor by interacting with and disrupting of integrins. The latter induces the reorganization of Tie2 that allows Ang2, an antagonist of Tie2 in the quiescent state, to function as an antagonist of Tie2 [ ]. The interaction of AXT107 with the integrins also leads to the reduction of the downstream signaling mediated by VEGF bound and its receptor. Co-regulation of the VEGF and Tie2 pathways could represent a viable option for promoting vascular integrity and decreasing vascular permeability and inflammation. An open-label, dose-escalating, 48-week, Phase 1/2a clinical study (CONGO) ( NCT04697758 ) was initiated in late 2020 to assess the safety and bioactivity of single intravitreal injection of AXT107, in approximately 18 subjects affected with DME.
Razuprotafib (AKB-9778) ( NCT03197870 ) is a small-molecule inhibitor of VE-PTP, which promotesTie2 activation, thus stabilizing ocular vasculature. A 48-week, randomized, double-masked Phase 2-b trial (TIME 2b) was conducted in eyes with moderate to severe NPDR to evaluate the safety and efficacy of twice-daily subcutaneous injection of AKB-9778 versus placebo. Although the study showed no safety issues, the primary endpoint in 2-step reduction in DRSS score was not met [ ].
Nesvacumab (REGN910-3) ( NCT02712008 ) is an anti-Ang2 monoclonal antibody that neutralizes Ang2 binding to Tie2 co-formulated with aflibercept for a single intravitreal injection. Anti-VEGF plus anti-Ang2 in fixed combination therapy in repeated doses was evaluated for DME in the 36-week Phase 2 study (RUBY), failing to demonstrate clinically significant visual acuity gains compared to aflibercept alone [ ].
Phase III development of razuprotafib and nesvacumab are not warranted after the respective phase 2 trials failed to meet their primary endpoints. To date, of the molecules targeting the angiopoietin/Tie pathway, only faricimab has progressed into Phase 3 study.
3.2
Longer lasting anti-VEGF drugs
3.2.1
Brolucizumab
Brolucizumab (Beovu; Novartis Pharmaceuticals, Basel, Switzerland), formerly known as ESBA1008, is a small single-chain antibody fragment that inhibits VEGF-A binding to its receptors, VEGF receptors 1 and 2. It has specifically been developed without the fragment crystallizable (Fc) domain in order to minimize the molecular size and to improve the affinity for its target, compared with the other anti-VEGF agents. Using pharmaceutical engineering, brolucizumab has reached the smallest molecular mass of the anti-VEGF molecules evaluated for human use, of approximately 26 kDa (kDa), for this reason, brolucizumab can be administered at a greater equivalent molar dose compared to other anti-VEGF agents in a single intravitreal injection [ ]. Based on these assumptions, it has been postulated that the molecule could have greater local efficacy, better durability of effect with fewer systemic side effects. However, data from previous registration trials in age-related macular degeneration (HAWK and HARRIER) showed that there is an increased risk in intraocular inflammation after brolucizumab treatment, with a rate of 4.6% versus 1.1% in aflibercept treated eyes [ , ]. These data seem to be confirmed by reports from its clinical use worldwide [ ].
KESTREL ( NCT03481634 ), KITE ( NCT03481660 ), and KINGFISHER ( NCT03917472 ) are three global, randomized, double-masked, phase III studies conducted to assess the noninferiority of brolucizumab 6 mg compared to aflibercept 2 mg in the treatment of DME. KESTREL and KITE are 2-year ongoing studies involving 926 patients in 36 countries. In both trials, subjects in the brolucizumab arms received 5 loading doses every 6 weeks followed by injections every 12 weeks, with an option to adjust the dosing every 8 weeks in presence of disease activity. The aflibercept groups were treated monthly for a total of five loading doses, in line with its label, followed by fixed dosing every 8 weeks. In both studies, the primary endpoint was met with respect to noninferiority in change in BCVA from baseline versus aflibercept after the first year of treatment. Patients treated with brolucizumab gained a mean of 10.6 letters and 9.2 letters versus 9.4 letters and 10.5 for patients treated with aflibercept in KITE and KESTREL, respectively. More than half of the subjects within the brolucizumab arm were maintained on a dosing interval of 12 weeks through the first year, following the loading phase. In addition, it showed a well-tolerated safety profile with an equivalent rate of intraocular inflammation compared to aflibercept [ ].
KINGFISHER is a 1-year, randomized, double-masked, Phase III trial, initiated in 2019 to evaluate in 517 patients at 115 centers the safety and efficacy of brolucizumab, dosed every 4 weeks, versus Aflibercept in patients with DME. KINGFISHER met its primary endpoint of noninferiority to aflibercept in change in BCVA from baseline with an overall well-tolerated safety profile [ ].
CONDOR ( NCT04278417 ) is a 96-week, randomized, multicenter, Phase III study conducted to assess efficacy and safety of brolucizumab versus PRP laser in patients with proliferative DR and is still in its recruiting phase.
3.2.2
KSI-301 (biopolymer conjugate)
KSI-301 (Kodiak Sciences Inc., Palo Alto, CA) is a humanized anti-VEGF IgG 1 monoclonal antibody conjugated with a high-molecular-weight phosphorylcholine-based polymer, which has been specifically engineered to increase the durability of anti-VEGF action [ ].
GLEAM ( NCT04611152 ) and GLIMMER ( NCT04603937 ) are two randomized, double-masked, multicenter, actively recruiting Phase III trials designed to evaluate KSI-3015 in terms of efficacy, durability, and safety versus Aflibercept in patients with treatment-naïve DME. In each study, experimental arms were given three monthly loading doses and then a personalized dosing regimen every 8–24 weeks, whereas comparator arms were given five loading doses and then every 8 weeks per its label. The primary outcome is to demonstrate that KSI-301 is noninferior to aflibercept with respect to mean change in BCVA at 1 year, and subjects will be treated and followed for 2 years.
3.2.3
Tyrosine kinase inhibition with GB-102
Sunitinib malate (Graybug Vision Inc., Baltimore, MD) is a small molecule inhibitor of tyrosine kinase receptor that can neutralize all VEGF receptor types, formulated in microparticle depot for intravitreal injection. The formulation consists of microparticles composed of poly-lactic-co-glycolic acid (PLGA) that aggregates to form a depot, which upon injection releases sunitinib malate over time and biodegrades into lactic and glycolic acid. The pharmacodynamics of GB102 is supposed to achieve sustained release of effective levels of sunitinib for many months [ ].
A multicenter, open-label, Phase IIa study ( NCT04085341 ) was initiated in September 2019 to evaluate the safety, tolerability, and pharmacodynamic response of a single intravitreal injection of either 1 or 2 mg depot formulation of sunitinib malate on subjects with DME that have received prior treatment with anti-VEGF.
3.2.4
Abicipar pegol
Abicipar-pegol (Allergan Inc., Dublin, Ireland), formerly known as DARPin ( d esigned a nkyrin r epeat p roteins) MP0112, is a recombinant protein coupled to a polyethylene glycol, which inhibits all isoforms of VEGF-A with high affinity. DARPins are genetically engineered single-domain proteins that mimic antibodies, binding selectively to a target protein with high affinity and specificity. Compared to antibodies, DARpins have smaller size, better bioavailability, more stability, and long intravitreal half-life [ , ].
Results from a multicenter, randomized, triple-masked, Phase II study ( NCT02186119 ) completed in 2015 showed that in 151 participants affected by DME, abicipar-pegol injected every 8 or 12 weeks allowed for less frequent injections with comparable results to monthly ranibizumab in terms of BCVA and CRT over a 28-week period [ ]. However, the high rate of intraocular inflammation has demonstrated an unfavorable benefit-risk ratio, leading to its withdrawal of the marketing authorization by Allergan in July 2020 [ ].
3.2.5
Conbercept
Conbercept (KH902; Chengdu Kanghong Biotech Company, Sichuan, China) is a recombinant fusion protein composed of the second domain of VEGFR1 and the third and fourth domains of VEGFR2 to the Fc region of human IgG 1 . It has a high affinity for all VEGF isoforms and for placental growth factor. It is similar to aflibercept from which it differs for the VEGFR2 portion, developed to potentially increase its efficacy and to produce affinity for VEGF-C. It binds all isoforms of VEGFA and VEGFB, and its pharmacokinetic profile has been developed to potentially increase the efficacy and affinity for its targets but with a lower dose [ ].
It was approved for treating neovascular AMD in China in 2013 [ ]. Since then, the molecule has been tested for other vascular retinal diseases, including DME.
The Sailing study (NCT02194634) is a 12-month multicenter, randomized, double-masked, Phase III trial, followed by a 12-month open-label extension study, performed in 18 centers in China designed to evaluate pro re nata (PRN) 0.5 mg conbercept intravitreal injections for the management of patients with DME versus laser photocoagulation. Results showed improvements in visual acuity and its efficacy was better than that of laser photocoagulation (8.2 ± 9.5 letters, P < .001 in the conbercept group versus 0.3 ± 12.0 letters, P = .810 in the laser group), meeting the primary endpoint [ ].
Fig. 19.2 summarizes important characteristics of some of the abovementioned drugs that are used to treat ocular angiogenesis.
3.3
Sustained release intravitreal implants
3.3.1
Port delivery system with ranibizumab
The port delivery system with ranibizumab (Hoffmann-La Roche, Basel, Switzerland) consists of an intraocular drug delivery system that allows a small, continuous release of ranibizumab into the vitreous cavity via passive diffusion. Therefore, the drug release is achieved over a longer time frame, with the primary intention of reducing the treatment burden. The device is a permanent, nonbiodegradable reservoir that is surgically implanted through a small incision in the sclera and pars plana. The reservoir is prefilled with 100 mg/mL of a customized formulation of ranibizumab, and can be refilled as needed in an office setting through the port’s self-sealing septum [ ].
Two Phase III studies on this device have been initiated for treating DR in 2019/2020:
The PAGODA trial ( NCT04108156 ) is a multicenter, randomized, Phase III study that will evaluate the efficacy, safety, and pharmacokinetics of the PDS in subjects with DME receiving the treatment with PDS implant refilled at fixed intervals every 6 months compared with monthly injections of ranibizumab 0.5 mg. The primary endpoint is to assess the change in BCVA from baseline to week 64. The estimated completion date for the trial is in late 2024.
The PAVILION trial ( NCT04503551 ) is a multicenter, randomized, Phase III study investigating the efficacy, safety, and pharmacokinetics of the PDS in subjects with DR and without center-involving DME. The primary endpoint is the percentage of participants with a ≥2-step improvement on the ETDRS-DRSS (diabetic retinopathy severity score) from baseline at week 52. PAVILION is actively recruiting, aiming for 160 patients.
The concept of surgically inserted drug depot is promising but requires good surgical skills of the ophthalmologist and does carry potential risks, including vitreous hemorrhage, cataract, and more severe retinal detachment, that must be weighed.
3.4
Gene therapy
3.4.1
RGX-314
RGX-314 (Regenxbio Inc., Rockville, MD) is being developed as a novel serotype 8 adeno-associated viral (AAV) vector that carries a gene expressing anti-VEGF monoclonal antibody fragment in targeted retinal cells through the proprietary gene delivery platform (NAV Technology Platform). The transduced protein fragment is supposed to curb the pathogenesis of the targeted DR by limiting abnormal blood vessel leakage and to deliver some advantages over conventional therapies, including a longer half-life thus a longer-lasting therapeutic effect [ ].
Positive data from preclinical studies and early-stage of clinical studies have furthered advancement to Phase II clinical study, ALTITUDE ( NCT04567550 ), to assess the efficacy, safety, and tolerability of RGX-314 in subjects with DR without center involved DME administered in suprachoroidal space. Forty participants with DR are being enrolled across two RGX-314 dose arms versus observation control with the primary endpoint being <SPAN role=presentation tabIndex=0 id=MathJax-Element-1-Frame class=MathJax style="POSITION: relative" data-mathml='≥’>≥≥
≥
2-step improvement in DR by ETDRS-DRSS at week 48.
3.4.2
ADVM022
ADVM-022 (Adverum Biotechnologies, Inc., Redwood City, CA) consists of an AAV.7m8 vector expressing aflibercept coding sequence for the treatment of retinal vascular diseases, including DME.
INFINITY ( NCT04418427 ) is a multi-center, randomized, double-masked, phase 2 trial in which 33 patients with DME are being treated with a single intravitreal injection of ADVM-022 in two dose cohorts, low (2 × 10 11 vg/eye) versus high (6 × 10 11 vg/eye), compared to intravitreal injection of aflibercept 2 mg. The primary objective is to evaluate the time to worsening of DME disease activity in the study eye. Interim data demonstrated that to date, 5 of the 12 patients treated with the high dose have experienced severe vascular diseases, leading the company to announce that it no longer plans future development for DME [ ].
3.5
Inhibiting different angiogenic/inflammatory pathways
3.5.1
Plasma kallikrein inhibitors
3.5.1.1
THR-149
THR-149 (Oxurion, Leuven, Belgium) is a bicyclic peptide inhibitor of plasma kallikrein, blocking the release of vasoactive and proinflammatory bradykinin in the vitreous, designed as a novel treatment for DME, and especially for those patients that lack response to the current standard-of-care therapy. Plasma kallikrein is a component of the plasma kallikrein-kinin (PKK) system, that has been shown to be upregulated in the diabetic human retina, leading to angiogenesis, local vascular permeability, and dilation, all of which make PKK system a potential target for treating DME [ ].
A Phase II, randomized, multi-center clinical trial KALAHARI ( NCT04527107 ) is ongoing with the aim to evaluate THR-149 in nonnaïve treatment DME patients. The study consists of two parts, dose-finding Part A that assesses three dose levels, and Part B evaluates the efficacy and safety of the selected optimal dose versus Aflibercept as comparator.
3.5.1.2
KVD001
KVD001 (KalVista Pharmaceuticals Inc., Massachusetts) is a highly selective plasma kallikrein inhibitor given by intravitreal injection, developed to evaluate poor-responder patients to anti-VEGF for the potential therapy of DME.
A randomized sham-controlled, double-masked, phase II proof-of-concept clinical trial ( NCT03466099 ) was conducted to assess the safety, tolerability, and efficacy of KVD001 for DME treatment. The study was completed in 2019, not meeting the primary efficacy endpoint of change in BCVA at 16 weeks compared to sham, even if “clinical benefit” on vision has been observed. No drug-related serious adverse events were observed. In relation to these findings, additional studies on plasma kallikrein inhibitors as methods to treat DME are warranted [ ].
3.5.2
RGD-integrin inhibitors
3.5.2.1
THR-687
THR-687 (Oxurion, Leuven, Belgium) is a pan-arginine-glycine-aspartic (RGD) acid integrin receptor antagonist that has a twofold action, inhibiting both upstream and downstream of VEGF, and therefore it attempts to have a broader efficacy than current anti-VEGF drugs for the treatment of DME [ , ].
Encouraging topline results in Phase I, dose-escalation study ( NCT03666923 ) to evaluate the safety of THR-687 administered by intravitreal injection for DME has prepared the molecule to enter a Phase II clinical trial.
3.5.2.2
ALG-1001
ALG-1001 (Luminate, Allegro Ophthalmics) is a synthetic oligopeptide that targets specific integrin receptors that have been shown to play a key role in the aberrant expression of blood vessels in DR.
A multicenter, phase 2 clinical trial (DEL MAR, NCT02348918 ) has been conducted to compare safety and efficacy of ALG-100, evaluated as a sequential therapy or combined with bevacizumab, compared to bevacizumab alone in 80 patients with DME. The primary endpoint of BCVA noninferiority was met at week 20, with a mean increase in BCVA of 7.1 letters in the group treated with ALG-1001 and bevacizumab compared to 6.7 letters in the bevacizumab-treated control group. Importantly, no adverse events occurred [ ].
3.5.3
Other molecules
3.5.3.1
OCS-01
OCS-01 (Oculis, Lausanne, Switzerland) is an innovative ophthalmic suspension of 1.5% dexamethasone developed on Soluble NanoParticle technology (SNP) under the property of Oculis to target DME. The formulation has been designed to enhance the bioavailability in the back of the eye of the topical treatment and to minimize the invasivity of ocular procedures.
The DX-211 study ( 2017-001172-36 ) is a multi-center, randomized, double-masked, phase 2 study. The objective of this study is to evaluate the efficacy and safety of dexamethasone nanoparticles eye drops in the improvement of visual acuity in participants with DME versus subjects receiving vehicle eye drops, 3 times per day for 12 weeks. The primary endpoint is the change in BCVA from baseline at 12 weeks and has been shown to be greater in the OCS-01 group than the vehicle one (+2.62 letters versus +1.04 letters, P = .125). No significant adverse events have been reported. Following the positive completion of Phase II study, OCS-01 is moving into Phase III study (DX211 Study, n.d.).
Table 19.1 summarizes the above described Phase II and III clinical trials for DME/DR and adds the most recent and rigorous trials in Phase I.