1
Introduction
It has long been well-established that the major complications of diabetes are microvascular complications such as nephropathy, retinopathy, and neuropathy, as well as the macrovascular complications including cardiovascular disease. More recently, epidemiologic data have shown that the skeletal system may be adversely impacted by diabetes leading to the subsequent development of diabetes-induced osteoporosis.
Osteoporosis is a systemic skeletal disease characterized by low bone mass or bone microarchitecture degradation that decreased bone strength and increased fracture risk. Both patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are at increased risk for fragility fracture although bone mineral density (BMD), the main determinate of bone strength, is slightly decreased in T1DM and is often normal or even increased in T2DM [ ]. This risk is increased at all ages in both male and female and increases dramatically with age, further above that of the general population [ ]. Bone turnover is decreased, and there is an alteration of the bone material properties and microstructure of bone in diabetes-induced osteoporosis; the latter particularly so when microvascular complications are present [ ].
There are complex pathophysiological mechanisms that explain bone fragility in DM and include chronic hyperglycemia, oxidative stress, and the accumulation of advanced glycation end products (AGEs) that compromise collagen properties, increase marrow adiposity, release inflammatory factors and adipokines from visceral fat, and potentially alter the function of osteocytes. In addition, some antidiabetic drugs can produce hypoglycemia that increase the risk of falls or increase bone fragility.
2
Fracture risk in diabetes
There is substantial evidence that the risk of fragility fractures is increased in both T1DM and T2DM patients. In the meta-analysis from Vilaca et al. [ ], the incidence of hip fractures in patients with T1DM reported a relative risk (RR) of 4.93 (95% confidence interval (CI) 3.06–7.95) and RR 1.33 (95% CI 1.19–1.49) in T2DM. The risk was greater in the younger population (<65 years) in both T1DM and T2DM. In T2DM, females, insulin users, and those with longer disease duration were at higher risk for hip fractures. The risk of nonvertebral fractures was 1.92 (95% CI 0.92–3.99) in T1DM while in T2DM, was 1.19 (95% CI 1.11–1.28). A meta-analysis of 25 cohort studies [ ] found an increase in the risk of all fractures (RR 1.32%, 95% CI 1.17–1.48), hip (RR 1.77%, 95% CI 1.56–2.02), upper arm (RR 1.47%, 95% CI 1.02–2.10), and ankle fractures (RR 1.24%, 95% CI 1.10–1.40) in both T1DM and T2DM. The risk of all ( P < .002), hip ( P < .001), and ankle fractures ( P = .029) was greater in T1DM patients comparing to T2DM patients. They also found a trend to greater risk for vertebral fractures (RR 1.56%, 95% CI 0.78–3.12), although it was not statistically significant. Although evidence regarding the risk of vertebral fractures in T1DM and T2DM remains inconclusive, according to the meta-analysis from Koromani and colleagues [ ], vertebral fractures seem to be more frequent in patients with T2DM (odds ratio [OR] 1.35, 95% CI 1.27–1.44) and Zhukouskaya et al. [ ] showed that T1DM patients have elevated prevalence of asymptomatic vertebral fractures independently of BMD. Therefore, DM has repeatedly been shown to be associated to an increased fracture risk, particularly marked at the hip.
Patients living with diabetes have impaired fracture healing because of bone metabolism alteration and microvascular disease. Additionally, they are at higher risk of complication such as infection, delayed wound closure, and perioperative cardiovascular events. These findings draw attention to the need for an improved awareness of the factors that determine risk of fracture in DM patients.
Several extraskeletal factors play a key role in the increased fracture risk observed in DM. DM patients have increased risk of falls because of hypoglycemic events, impaired balance, and low muscle mass and strength that are well-known risk factors for falls [ ]. Besides, those treated with insulin have a particularly high risk of falls [ , ] maybe by the fact that patients with DM who are treated with insulin usually have more severe or longer duration disease with an increased risk of diabetic complications such as visual defects, impaired renal function, and peripheral neuropathy. Table 11.1 summarizes the main fracture risk factors associated to DM.
| T1DM | T2DM | Both T1DM and T2DM |
|---|---|---|
|
|
|
3
Mechanisms underlying bone loss and fragility
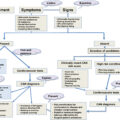
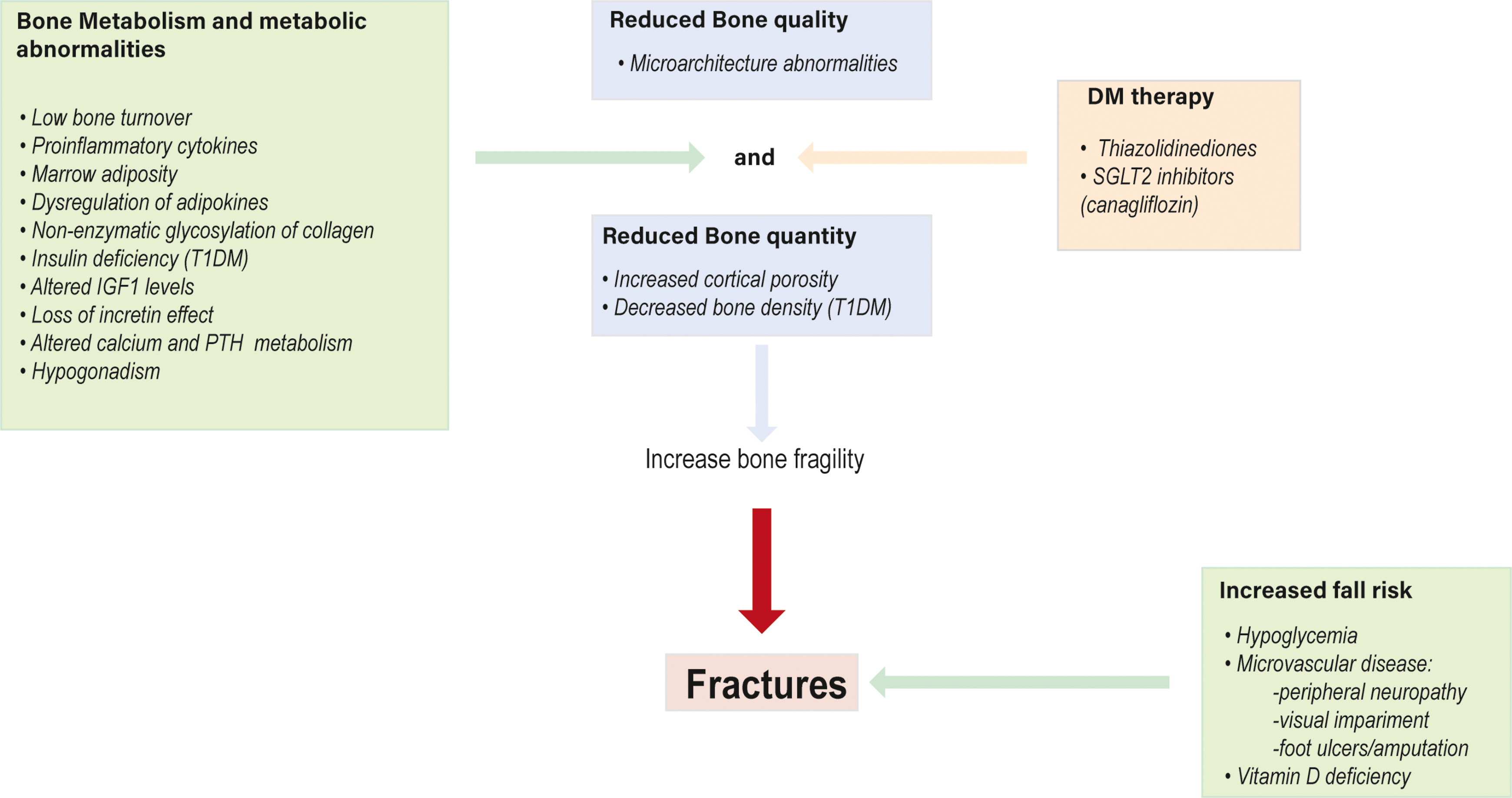
The mechanisms by which diabetes increases skeletal fragility are complex and differ between T1DM and T2DM, although both involve relatively reduced bone formation, osteoblast dysfunction, and low bone turnover. Fig. 11.1 represents the main mechanisms involved in bone fragility.
3.1
Bone strength
Bone fragility consists of decreased bone mineral mass and alterations in bone microstructure and, eventually, in the intrinsic properties of the bone material itself. BMD measures are the gold standard to predict osteoporosis and osteopenia, although tend to underestimate the fracture risk, particularly in type 2 diabetes [ ].
T1DM has decreased BMD although is not decreased to a level that explains the observed fracture risk. The presence of microvascular disease has been suggested to be associated to the decreased BMD observed in patients with T1DM although it is not clear if this risk is related to the duration of disease and metabolic control [ , , ] because it has been reported that T1DM individuals have low bone mass at the time of diagnosis [ ]. This could be explained by the fact that the lack of insulin and low levels of insulin-like growth factor 1 (IGF1) in T1DM suppresses the terminal mesenchymal stem cells differentiation (MSCs) into osteoblasts and inhibits skeletal growth, which are difficult to achieve an adequate peak bone mass at a young age [ ]. On the contrary, T2DM patients have high risk of fracture despite of a normal or even increased BMD. This contradiction can be explained by impairments of bone architecture and biomechanical properties that may be negatively affect by many factors such as insulinopenia, hyperglycemia, the development of AGEs, chronic inflammation, and microvascular disease ( Fig. 11.2 ) [ , ]. Besides, the development of noninvasive imaging techniques has shown that T2DM patients have higher trabecular volume and an increase in intracortical porosity [ ] and are suggested to have higher bone marrow adiposity [ ] explaining in part the reduction in bone strength.
3.2
Bone metabolism
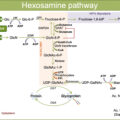
Hydroxyapatite and interconnecting collagen fibers are the main components of extracellular bone matrix. Hydroxyapatite provides stiffness, and collagen fibers provide tensile strength and counteracts shear stresses [ ]. Cellular activity, bone turnover, and collagen cross-link formation regulate these material properties of bone tissue [ ]. Besides, many environmental factors, such as circulating hormones, glucose toxicity, oxidative stress, Wnt pathway inhibition, and level of glycation, impair the mechanostatic function of osteocytes, bone turnover, and collagen properties [ ].
3.2.1
Cellular activity
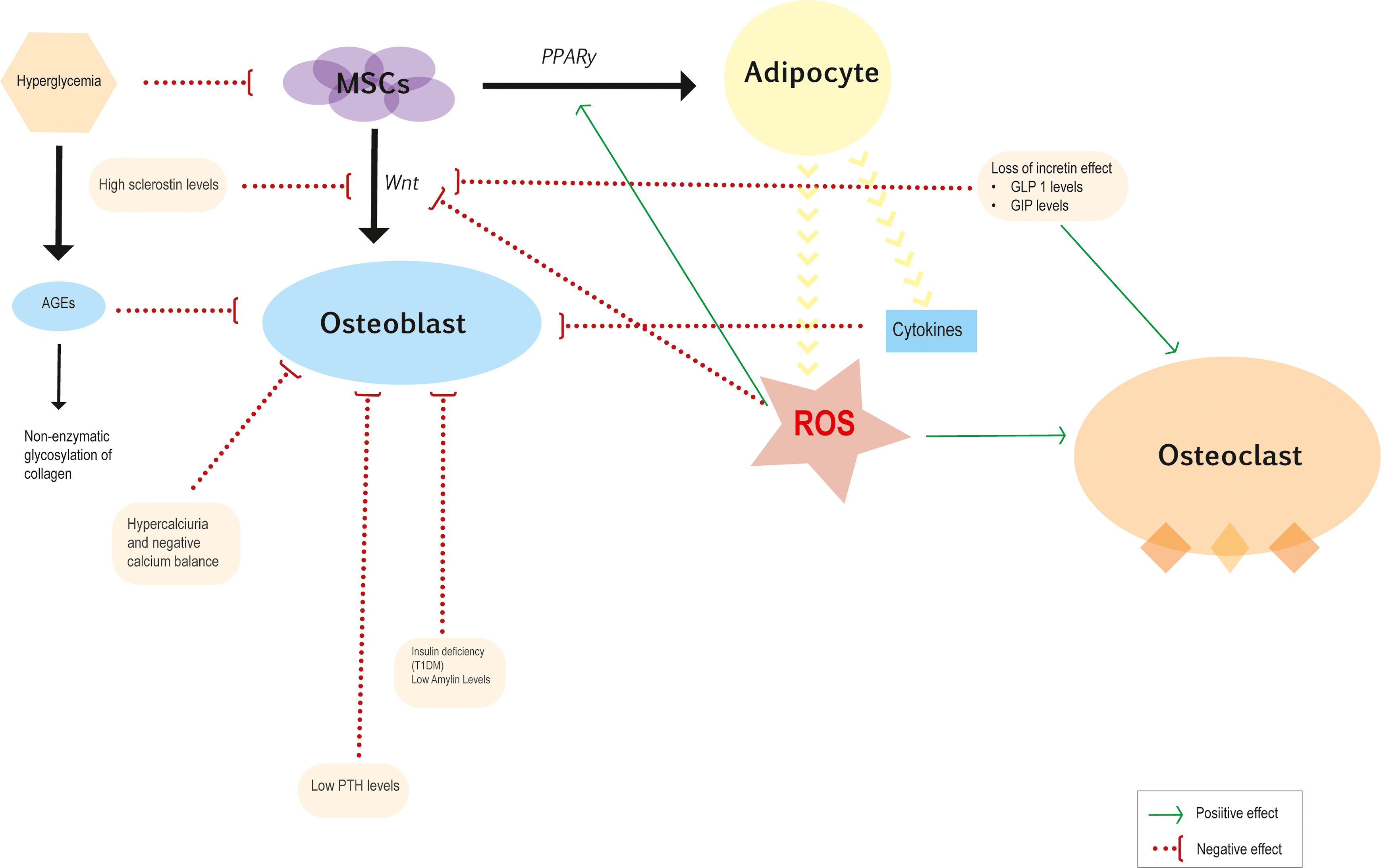
The MSC is common precursor for adipocytes and osteoblasts. The activation of the Wnt signaling pathway facilitates the differentiation of MSCs into osteoblasts and inhibits adipogenesis, while the peroxisome proliferator-activated receptor gamma (PPAR-γ) pathways promote adipogenesis [ ]. Hyperglycemic environment has been related to increase adipogenesis markers and downregulate osteoblast differentiation [ , ]. Additionally, hyperglycemia has been shown to increase production of sclerostin, which induces adipogenesis by inhibiting Wnt signaling [ ].
Obesity and diabetic environment leads to increased levels of inflammatory cytokines, in particular TNF-α, IL-1, and IL-6 and reactive oxygen species (ROS), which induce osteoblast apoptosis and facilitate MSC differentiation into adipocytes. This leads to a vicious cycle of metabolic stress, which upholds a chronic inflammatory process that may deteriorate trabecular bone and has a direct impact on the differentiation and function of MSCs, osteoclasts, osteoblasts, and osteocytes [ ].
Increased bone marrow adiposity is suggested to be a mechanism of diabetic fragility fractures [ , ]. In bone marrow, adipocytes generate free fatty acids that release ROS, which induce osteoblast apoptosis. A negative association between marrow adipose tissue (MAT) and BMD has been noted in overweight, postmenopausal women with T2DM [ ]. However, there is currently no evidence that suggests that DM directly explains increased bone marrow adiposity and its implications for the structural integrity of the skeleton in DM remain to be elucidated. On the other hand, brown adipose tissue has been found to be inversely associated with obesity and T2DM [ ]. Insulin-like growth factor-binding protein 2 and Wnt10b, are anabolic factors secreted by thermogenically active brown adipose tissue, that induce osteoblast activity, and may be beneficial for the skeleton [ ].
Osteoblasts regulate osteoclastogenesis by producing the receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG). Levels of OPG are associated with fat mass and atherosclerosis parameters in diabetes, and RANKL have been shown to participate in the genesis of insulin resistance [ ]. The effect of DM on osteoclastic function and differentiation is controversial. Some studies have suggested increased osteoclastic activity in DM, especially in periodontal disease [ ] and osteoporosis [ ] while others suggest inhibited osteoclast function [ ]. Thereby, it seems that the impaired bone remodeling in DM is primarily due to inhibited osteoblastic and progenitor cell activity rather than an alteration of osteoclastic activity.
3.2.2
Bone turnover
The effect of a diabetic environment on bone metabolism can be indirectly measured through bone turnover markers. Patients with diabetes typically have low bone turnover with reduction in bone formation and, to a lesser degree, bone resorption.
Osteoblasts produce osteocalcin, that is a marker of bone formation; serum concentrations of osteocalcin were found to be lower both in patients with T1DM [ ] and T2DM than in individually matched controls [ ]. Osteocalcin had also been related to glucose homeostasis, stimulating beta cells proliferation and insulin secretion in vitro and animal studies, while human studies have shown conflicting results [ ]. In T1DM children, osteocalcin was negatively correlated with HbA1c [ ]. On the other hand, studies evaluating treatments or conditions which are able to change osteocalcin levels have shown opposite results. Alendronate therapy, which decrease osteocalcin levels, was associated with reduced diabetes risk [ ].
Alternatively, sclerostin, an endogenous inhibitor of the Wnt signaling pathway, was found to be higher in patients with T2DM and was associated with a decrease in bone turnover markers for bone formation [ , ]. In addition, high sclerostin and dickkopf-1 (DKK-1) serum levels were found elevated in children and adolescents with T1DM [ ]. Thus, osteocytes alterations in DM could explain low bone formation rate and potentially decreased bone quality.
C-terminal telopeptide of type I collagen (CTX), a marker of bone resorption, was decreased, and alkaline phosphatase (ALP) was increased in a meta-analysis assessing bone turnover in DM [ ]. Others bone turnover markers such as procollagen type 1 amino-terminal propeptide (P1NP), N-terminal telopeptide of type I collagen (NTX), and deoxypyridinoline also tended to be lower in patients with DM than in nondiabetic controls [ ].
Indirectly, other additional factors may induce a low turnover state. Dysregulation of adipokine levels could partly explain the alterations in bone turnover. Levels of adiponectin, produced by adipose tissue, have been shown to be lower in patients with T2DM [ ], and low levels of leptin, another adipokine produced by white adipose tissue, bone marrow adipocytes, and osteoblastic cells were also found in patients with DM [ ]. The effect of adipokines on bone metabolism is complex and has contradictory results. Some studies are in favor of an inverse relationship between adiponectin and bone mass [ , ] while others not [ ]. According to some studies, increased leptin levels have been suggested to be protective against fractures, while others studies have not found any relationship [ ]. Additionally, irisin, a specific adipo-myokine, may promote osteogenic differentiation, increase in cortical bone mass and strength, and reduce osteoclastogenesis [ ]. DM patients may loose of all these positive effects on bone mass because of irisin resistance [ ].
In summary, most of the recent studies have confirmed decreased levels of bone turnover markers in patients with diabetic mellitus and may be useful in the evaluation of bone metabolism in diabetic patients.
3.3
Metabolic abnormalities
3.3.1
Hyperglycemia and oxidative stress
Hyperglycemia and oxidative stress increase levels of AGEs [ ] such as pentosidine. Activation of the receptor for AGEs (RAGE) favors inflammatory cytokine and ROS production that interfere with osteoblastic and osteoclastic activity [ ]. Besides, accumulation of AGEs alters the structural integrity of the collagen matrix and negatively affects biomechanical properties of cortical and trabecular bone [ ]. AGEs cros-link between collagen fibers leads to more brittle bones that are prone to fracture, whereas normal enzymatic cross-linking in collagen shows a beneficial effect on the quality and strength of the bone [ ]. Patients with diabetes have elevated levels of AGEs, such as pentosidine, which is associated with an increased risk of vertebral fracture [ ]. These adverse effects of AGEs on bone contribute to enhance bone fragility.
Chronic hyperglycemia has a direct and indirect deleterious effect on bone. Hyperglycemia interfering affects osteoblast by downregulating expression of the osteocalcin and facilitating the differentiation of MSCs into adipocytes [ ]. This shift to an adipogenic lineage is mediated through the production of ROS [ ]. However, the level of hyperglycemia at which bone is adversely affected is not clear and studies that examine the relationship between fracture risk and glycemic control are inconclusive. It is suggested that a target HbA1c level of <7.5% could reduce fracture risk in patients with DM [ , ].
3.3.2
Insulin, IGF1, and amylin
Insulin acts as an anabolic factor on bone and exerts direct action in osteoblasts by activation of its cell surface receptor. IGF1 modulates the strength of the insulin-generated signal through interactions with the IGF1 receptor (IGF1R) [ ]. T1DM is typically characterized by insulinopenia in combination with low levels/low action of IGF1; this condition has an inhibitory effect on osteoblasts and their progenitor cells in the early stages of the disease, leading to a low bone formation [ , ]. However, in T2DM, insulinopenia and low levels of IGF1 would be expected in advanced stages of the disease, and the main defect would be insulin resistance. Previous studies have described the deleterious effect of insulin resistance on bone strength and bone quality in T2DM [ , ], although how insulin resistance can affect bone has not been well established. Supraphysiological insulin levels seem to be associated with reduced bone turnover. Although reduced bone turnover may increase BMD, it may also contribute to bone fragility through increases in cortical porosity or other deficits in bone microarchitecture. In addition, other hormones that are involved in the regulation of glucose metabolism such as IGF1, adiponectin, and incretins may contribute to the effects of insulin resistance on bone [ ]. There is also a deficiency of amylin in T1DM. Although high levels have been shown to correlate with higher bone mass, there are contradictory results from studies exploring the osteogenic effect of amylin [ ].
3.3.3
Effect of incretin hormones
Gastric inhibitory polypeptide (GIP) and glucagon-like peptide 1 (GLP1) are incretins hormones secreted in the jejunum and distal ileum, respectively. In animal models, incretins are thought to have an osteogenic effect. There are GLP1 receptors on bone marrow stromal cells where inhibit their differentiation into adipocytes and stimulate Wnt pathway. The net effect of these animal experiments is that incretin hormones increase bone formation and bone mineral density and improve bone strength [ , ]. Besides, GLP1 receptors are also expressed on thyroid C cells and, therefore, increase the secretion of calcitonin, which could contribute to the postprandial decrease in bone resorption [ ]. The role of endogenous incretins in diabetic bone may be altered because T2DM patients have a reduced incretin effect with impaired GLP1 production or action [ ]. Further clinical and nonclinical studies are needed to determine the effects of incretin hormones on bone metabolism.
3.3.4
Calcium and PTH metabolism
Hypercalciuria and a negative calcium balance is a common finding in uncontrolled diabetes. Furthermore, negative calcium balance is associated with an inappropriate PTH response [ ]. Relative hypoparathyroidism in diabetics patients could contribute to low bone turnover. Reduced serum levels of CTX and tartrate resistant acid phosphatase 5b (TRAP5b) have been found to correlate with low levels of PTH [ ]. A dysfunction of calcium sensing receptors or associated hypomagnesemia may be factors involved in the alteration of PTH secretion [ ].
Both obesity and DM patients have impaired vitamin D status. In addition to its effects on bone, vitamin D could participate in the maintenance of glycemic control, since pancreatic beta cells express vitamin D receptors. However, vitamin D supplementation has not consistently been shown to improve glycemic control in patients with diabetes [ ]. Moreover, vitamin D supplementation in young patients with new-onset T1DM did not improve markers of bone turnover [ ].
In another way, renal impairment with osteodystrophy is a major complication in advanced diabetes that may disturb calcium, phosphate, FGF-23, and vitamin D physiology and may have a major impact on the bone diabetes disease.
The cellular and molecular mechanisms underlying bone fragility in DM are shown in Fig. 11.2 .
3.4
Microvascular disease
The association between microvascular disease and bone microstructure as well as with fracture risk observed in some studies suggests that an altered bone microvasculature could have a role in compromising bone formation. It is unclear whether bone deficits in patients with microvascular disease are caused by a microangiopathy leading to abnormal blood flow and altered bone remodeling, changes in vascular endothelial growth factor signaling, or due to the increased accumulation of AGEs, either directly affecting bone cells or the inherent material property of bone tissue due to the deposition into bone collagen or indirectly eliciting vascular damage [ ]. Microvascular disease may affect the bone marrow microenvironment where bone progenitor cells reside, with shifts in production to adipocytes and away from osteoblasts, resulting in an increase in bone marrow adiposity [ ]. Oikawa et al. found basement membrane thickening, capillary rarefaction, and apoptosis in the bone marrow vasculature in a mouse model of diabetes [ ]. These morphological changes are similar features to other typical diabetic microvascular complications.
There are discrepancies in the bone microarchitecture in patients with type 1 and type 2 diabetes and microvascular disease. T1DM patients had alterations in the trabecular and cortical compartments, whereas those with T2DM had deficits in the cortical compartment, with absence of defects in the trabecular compartment. It is possible that the preferential involvement of the cortical compartment in patients with microvascular disease could be related to a possible deficiency of pericytes in cortical bone, that are the main and only source of osteoprogenitors in remodeling, while the trabecular bone has other alternative osteoprogenitor sources such as lining cells situated at quiescent bone surfaces adjacent to the bone surface undergoing remodeling and canopies, which represent the mesenchymal bone marrow envelope above bone remodeling sites [ ]. Hyperglycemia and AGEs are common factors both in T1DM and T2DM and involved in bone diabetes disease and do not seem to explain the discrepancies in the bone microarchitecture and microvascular disease. Nevertheless, there are three key differences between the two types of diabetes might explain this discrepancies. First, the age of onset of diabetes, T1DM, is commonly diagnosed at childhood or adolescence, while T2DM is onset at old age. Second, the main effect in T1DM is insulin deficiency while in T2DM predominate hyperinsulinism, and finally, obesity is most common in T2DM than in T1DM patients.
Emerging evidence from longitudinal studies has shown that there is dominance of cortical over trabecular bone loss at the peripheral sites [ ]. These patterns of normal age-related changes to bone microarchitecture are pertinent in the context of the potential skeletal effects of the age of onset of diabetes and the appearance and development of diabetic microvascular disease.
In addition, microvascular complications, such as neuropathy and retinopathy with visual impairment, increase the risk of falling and subsequent fractures. Neuropathy may also cause localized bone loss, which may increase the risk of fracture at the foot and ankle.
3.5
Effect of antidiabetic drugs on the risk of fracture
The effect of antidiabetic drugs on bone metabolism is complex and is not always clear. The antidiabetic drugs can play an important role on the modulation of bone metabolism affecting the risk of fragility fractures ( Table 11.2 ). The increased risk of fall associated with hypoglycaemic agents and diabetic complications may act as confounding factors for the bone-specific effect associated with antidiabetic therapy.