1
Introduction
Evidence is accumulating that type 2 diabetes (T2D) is associated with cognitive impairment and dementia, and numerous epidemiological studies have demonstrated that T2D patients have a significantly higher risk of developing dementia [ ], in particular Alzheimer’s disease (AD) [ , ]. AD is the most common cause of dementia [ ] and is characterized by a progressive amnestic disorder with subsequent appearance of other cognitive, behavioral, and neuropsychiatric changes that impair social function and activities of daily living [ ].
Thus, cognitive impairment and dementia are emerging as significant comorbidities of T2D, with important implications for clinical practice and funding of public healthcare systems. An additional concern in T2D is that cognitive impairment affects the required complex self-management of diabetes (e.g., medication, diet, blood glucose monitoring). In addition, T2D subjects with dementia are at greater risk of hypoglycemia than older people without dementia. For all these reasons, T2D subjects with cognitive impairment present an increased frequency of hospital admissions and morbi-mortality [ , ]. Unfortunately, in clinical practice, there are no reported phenotypic indicators or reliable examinations to identify T2D patients with mild cognitive impairment (MCI), despite the fact that recent guidelines do recommend screening for cognitive impairment in T2D [ , ]. MCI describes a transitional state between normal cognitive function and dementia. Individuals with MCI have a degree of memory loss with little impact on their daily activities, and its diagnosis is based on complex neuropsychological tests [ ], which makes their incorporation into current standards of care for the T2D population unfeasible. For this reason, a multidisciplinary, large-scale approach, which integrates clinical and molecular information, is urgently needed to identify T2D patients at risk of MCI and dementia and also to identify new opportunities for personalized management.
In recent years, growing evidence has shown that retinal neurodegeneration is an early event in the pathogenesis of diabetic retinopathy (DR) [ , ]. In addition, an association between retinal vessel abnormalities and cognitive impairment and dementia has been well-documented [ , ]. Since the retina is ontogenically a brain-derived tissue, it has been suggested that it may provide an easily accessible and noninvasive way of examining the pathology of the brain: “The eye as a window to the brain” [ , , ]. Therefore, it seems reasonable to propose that the evaluation of retinal parameters related to either neurodegeneration or microvascular disease would be robust and valuable biomarkers to identify those T2D patients at higher risk of developing cognitive impairment and dementia.
2
Epidemiological evidence
T2D and AD are two prevalent diseases associated with a significant economic burden for the healthcare systems of developed countries. Both diseases are age-related and, therefore, it is not surprising that they run in parallel and are particularly prevalent in the developed world.
In recent years, there is mounting evidence that T2D is associated with cognitive impairment and dementia, and numerous epidemiological studies have demonstrated that T2D patients have a significantly higher risk (∼2-fold higher) of developing AD in comparison with age-matched nondiabetic subjects [ , ]. This increased risk is maintained even after adjusting for cardiovascular risk factors [ , ]. In addition, T2D patients have an increased risk of developing MCI [ ] as determined using standard tests, which detect early-stage deficits which, as yet, have had no impact on activities of daily living. This represents a critical transitional state between normal cognitive function and dementia, especially since the annual conversion rate from MCI to dementia ranges between 10% and 30% in the general population [ ]. We have reported that T2D acts as an important accelerator of dementia in patients with MCI [ ].
MCI is a clinically heterogeneous syndrome. Its first classification was based on memory impairment, but it was later expanded to other cognitive domains. According to Petersen et al. [ ] classification, MCI would comprise four broad subgroups depending on memory performance and the number of impaired cognitive functions: amnestic single and multiple domains, and nonamnestic single and multiple domains. Some recent studies suggested that the risk of AD increases when additional domains besides memory are impaired, probably because they are in a more advanced stage of the neurodegenerative disease [ ].
Over the last decades, most research on MCI has been focused on searching for factors which make patients more vulnerable to conversion to dementia, in particular AD. Ciudin et al. have previously reported that T2D acts as an important accelerator of dementia in patients with MCI [ ]. The patients T2D with MCI who converted to dementia presented a higher prevalence of APOEε4 allele and DR than T2D nonconverters. The presence the ε4 allele APOE may be associated with accelerated neurodegeneration in the development and progression of neurodegenerative diseases [ , ]. This also points out the importance of improving diagnosis and follow-up of all the patients with MCI [ ]. Furthermore, APOE ε4 is the strongest genetic risk factor for sporadic AD. Thus, it is no surprise that the presence of even a copy of the εallele shortens the time of progression to dementia.
Although the APOE4 genotype is a well-recognized major determinant of developing AD, the role of DR as a risk factor for cognitive decline has been less recognized. The ACCORD-MIND [ ] and ACCORD-Eye sub-studies [ ] showed that DR is associated with lower gray matter volume and predicted future cognitive decline in the T2D population. In addition, it was recently reported that T2D patients with advanced DR presented a 42% higher risk of incident dementia [ ]. Ciudin et al. recently showed the first evidence that DR is a risk factor for conversion from MCI to dementia in T2D patients [ ]. Therefore, both APOEε4 allele and the presence of DR might be contemplated as a herald of dementia risk in MCI patients, in particular AD.
The impact of dementia on affected individuals and society as a whole is huge 604 billion US$ in 2022 [ ] and the number of cases of dementia associated with T2D is expected to increase because of the diabetes pandemic and the concomitant rise in aging populations worldwide.
3
Molecular pathways linking T2D and AD
Alzheimer’s disease is thought to be the main cause of dementia in subjects with T2D, and it has been proposed that T2D is an accelerator of cognitive decline [ ]. However, cognitive decline in T2D does not always strictly link to the definitive pathologies observed in AD. The cardinal pathological features of AD have been known for more than 100 years, and today, the postmortem presence of amyloid plaques and neurofibrillary tangles (NFT) are still required for a pathological diagnosis [ ]. Takenoshita et al. [ ] explored amyloid and tau load in 31 patients with T2D and showed that 81% of the patients were tau positive, but only 39% of the patients were also Aβ positive. In addition, higher levels of tau and phosphorylated tau have been reported in the cerebrospinal fluid (CSF) of patients with T2D in comparison with nondiabetic patients [ ]. Animal models of dementia mostly rely on AD-specific pathologies [i.e., Aβ, amyloid precursor protein (APP), tau], whereas models for studying the prodromal MCI stage preceding dementia are lacking [ ].
AD and T2D share similar progressive vasodegeneration, glial activation, and neural damage, which arise from impairment of insulin signaling, the presence of low-grade inflammation, and the activation of pathways directly related to chronic hyperglycemia. Therefore, it is useful to draw comparisons between these commonly affected pathways in both the retina and the brain in order to understand cognitive decline in T2D.
3.1
Impairment of insulin signaling
The impairment of insulin signaling (leading to insulin resistance) in the brain plays an essential role in the pathogenesis of AD and precedes cognitive dysfunction and pathological alterations even by decades ( Fig. 14.1 ). For this reason, AD is so called “type 3 diabetes” or a state of “brain insulin resistance.”
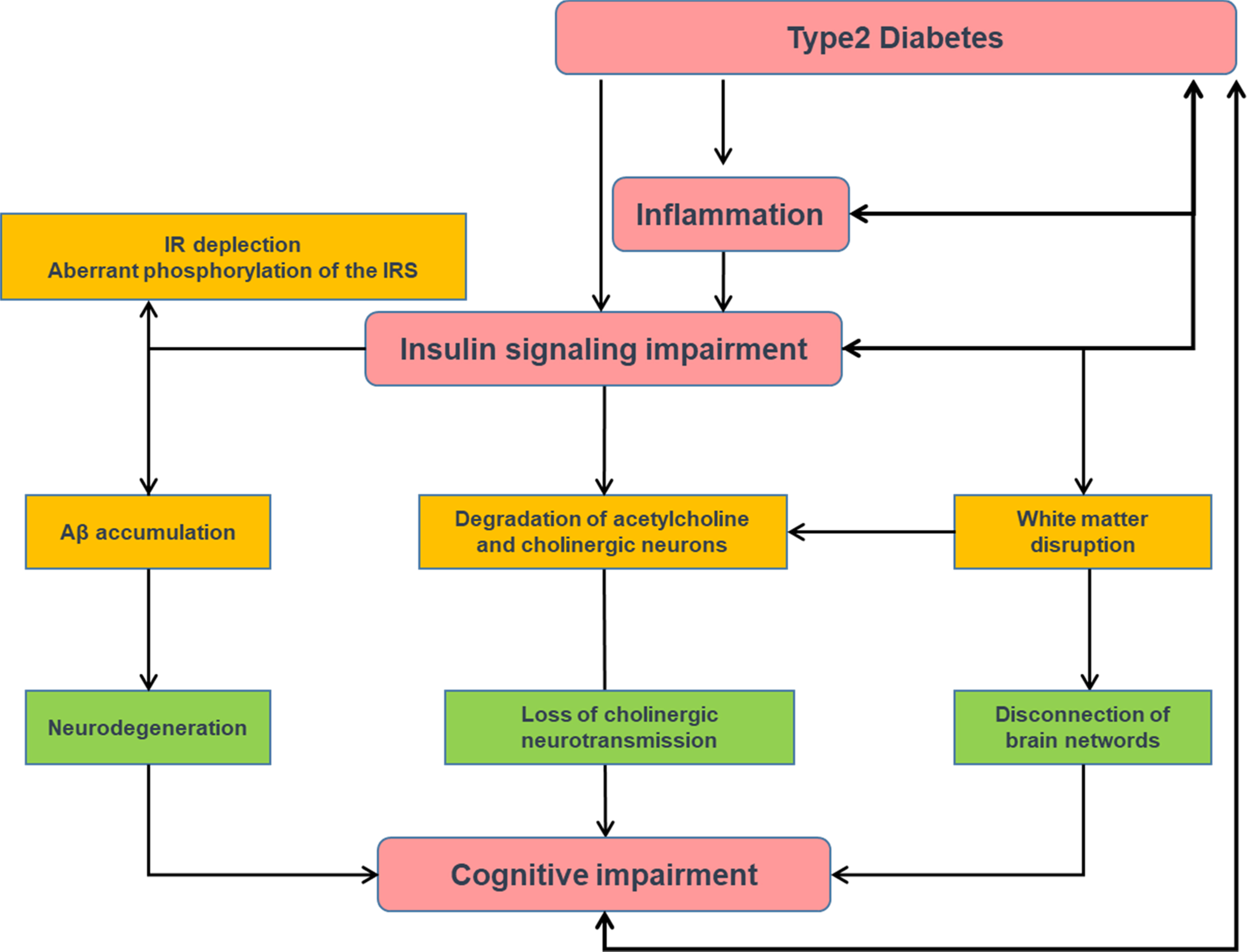
Insulin signaling is essential for neuronal survival, as well as for learning and memorizing. In fact, in animal models, impaired insulin signaling precedes Aβ accumulation [ ]. Numerous studies have proposed that degeneration of cholinergic neurons and the loss of cholinergic neurotransmission in the brain lead to AD [ ]. Insulin resistance may play an important role in cognitive impairment due to a metabolism disorder of the cholinergic system of the brain. Basic research showed that insulin signaling-related proteins are co-located with cholinergic neurons in the hippocampus [ ], thus suggesting that insulin plays a role in the regulation of the metabolism of the acetylcholine. Insulin resistance contributes to the degradation of acetylcholine and cholinergic neurons degeneration, which are involved in the onset of the cognitive impairment [ ].
Additionally, several studies have reported that insulin resistance may lead to axonopathy and cytoskeletal disruption, and dysregulation of white matter synaptic transmission which play an important role in the process of AD [ , ]. It has been recognized that the loss of the integrity of synapses often precedes neuronal death [ ]. Furthermore, the disruption of axonal transport seems to play a role in the decreased synthesis and storage of acetylcholine in the vesicles, a process, which is important for learning and memory [ ].
One mechanism by which there is an impairment of insulin signaling in the brain of AD is due to Aβ oligomers, which effectively deplete insulin receptors from surface membrane of neurons, thereby contributing to insulin resistance [ ]. In addition, an aberrant phosphorylation of insulin receptor substrate (IRS) exists in the brains of patients with AD, whereas the normal phosphorylation of the insulin receptor is dramatically reduced. This phenomenon was also shown by culturing hippocampal neurons with Aβ oligomers [ ]. This impairment in insulin signaling reduces normal prosurvival signaling in neurons and promotes their death by apoptosis.
Apart from an impairment of insulin signaling, a deficit of brain insulin production also exists. The pancreas is not the only organ that produces insulin as lower quantities of this protein are also produced by the brain, which provides critical paracrine signals for neuron survival. Interestingly, it has been shown that both insulin and insulin receptors are less expressed in the hippocampal region from patients with AD than in subjects without AD [ ]. Both the impairment of insulin signaling and insulin-deficiency lead to tau phosphorylation, and this results in tau aggregates, which are essential for the structural neurodegenerative process. In addition, these tau aggregates accelerate oxidative stress, neuroinflammation, and mitochondrial dysfunction, thus worsening the process. Brain insulin resistance also favors the production of Aβ, which may form oligomers and plaques that contribute to insulin resistance and tau phosphorylation, thus closing a vicious circle [ ].
Su et al. [ ] identified that neurobiological alterations related to brain insulin resistance modify AD progress by influencing the overall efficiency of brain functional networks. The MCI patients showed disconnections of the brain networks mainly in the cerebellum-frontal-temporal regions. Additionally, the brain network alteration induced by the insulin resistance partially determined the longitudinal cognitive decline.
Interestingly, intranasal insulin (which provides rapid delivery of insulin to the central nervous system (CNS) via bulk flow along olfactory and trigeminal perivascular channels and avoid hypoglycemia) has demonstrated to be effective in arresting the cognitive decline in amnestic MCI and mild to moderate AD [ ]. Current research is focusing on pancreatic islet transplantation into the CNS, with encouraging results in murine models [ ].
3.2
Inflammation
Inflammation is also a key pathogenic factor of AD. In insulin-resistance conditions, such as obesity and T2D, there is an increased inflammatory state typified by immune cell and vascular endothelial activation, and increased levels of circulating proinflammatory cytokines, which are able to cross the blood–brain barrier (BBB) and reach their neuronal receptors. The activation of these receptors induces signaling through stress kinases which, in turn, leads to brain insulin resistance. In addition, Aβ oligomers also activate stress kinases and mediate microglial activation, thus exacerbating the inflammatory process [ ] ( Fig. 14.2 ).
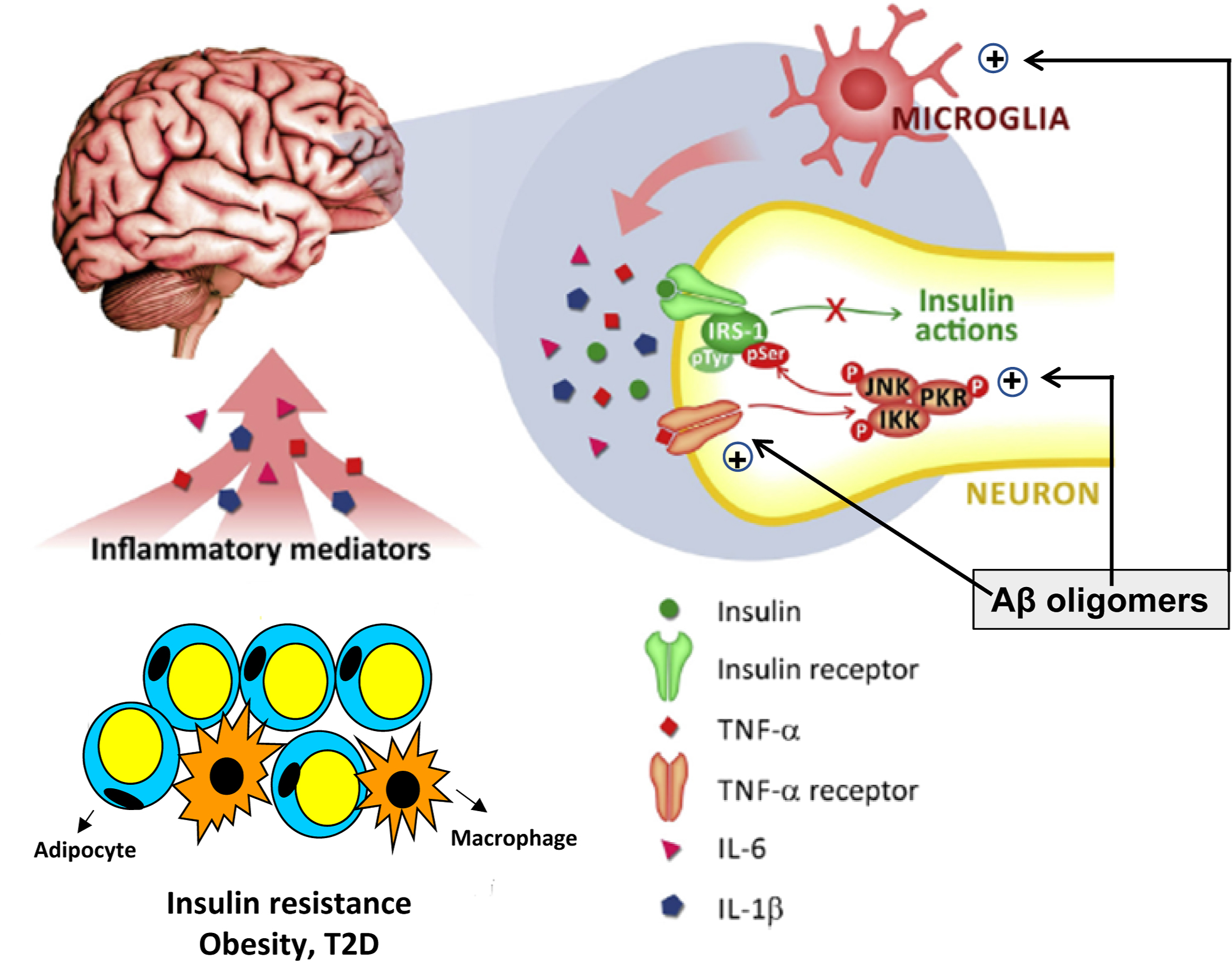
Several studies have linked high blood levels of inflammatory markers, such as C-reactive protein, tumor necrosis factor alpha (TNF-α), and interleukin-6 (IL-6), with cognitive disfunction [ ]. In addition, Guo et al. [ ] have reported that reducing the levels of NF-κB p65, iNOS, TNF-α, and IL-1β in the hippocampi of APP/PS1 transgenic mice might revert the cognitive deficits.
3.3
Advanced glycation end-products
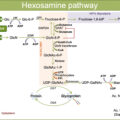
Advanced glycation end-products (AGEs) also accumulate in nondiabetic patients and exert deleterious effects by multiple and complex pathways [ ]. In this regard, it should be emphasized that Aβ (and other pro-inflammatory proteins such as S100B and HMGB1) are activating ligands for the key innate immune response receptor receptor for advanced glycation end products (RAGEs), even in nondiabetic patients.
Some studies have suggested that RAGE might participate in the lesions of the brain in AD through neuroinflammation triggered by activation of RAGE signaling [ ]. In addition, T2D patients with MCI have significantly higher serum levels of AGEs and RAGE than the T2D patients without cognitive impairment [ ].The main mechanisms by which RAGE activation participates in the development of AD are summarized in Fig. 14.3 [ ].
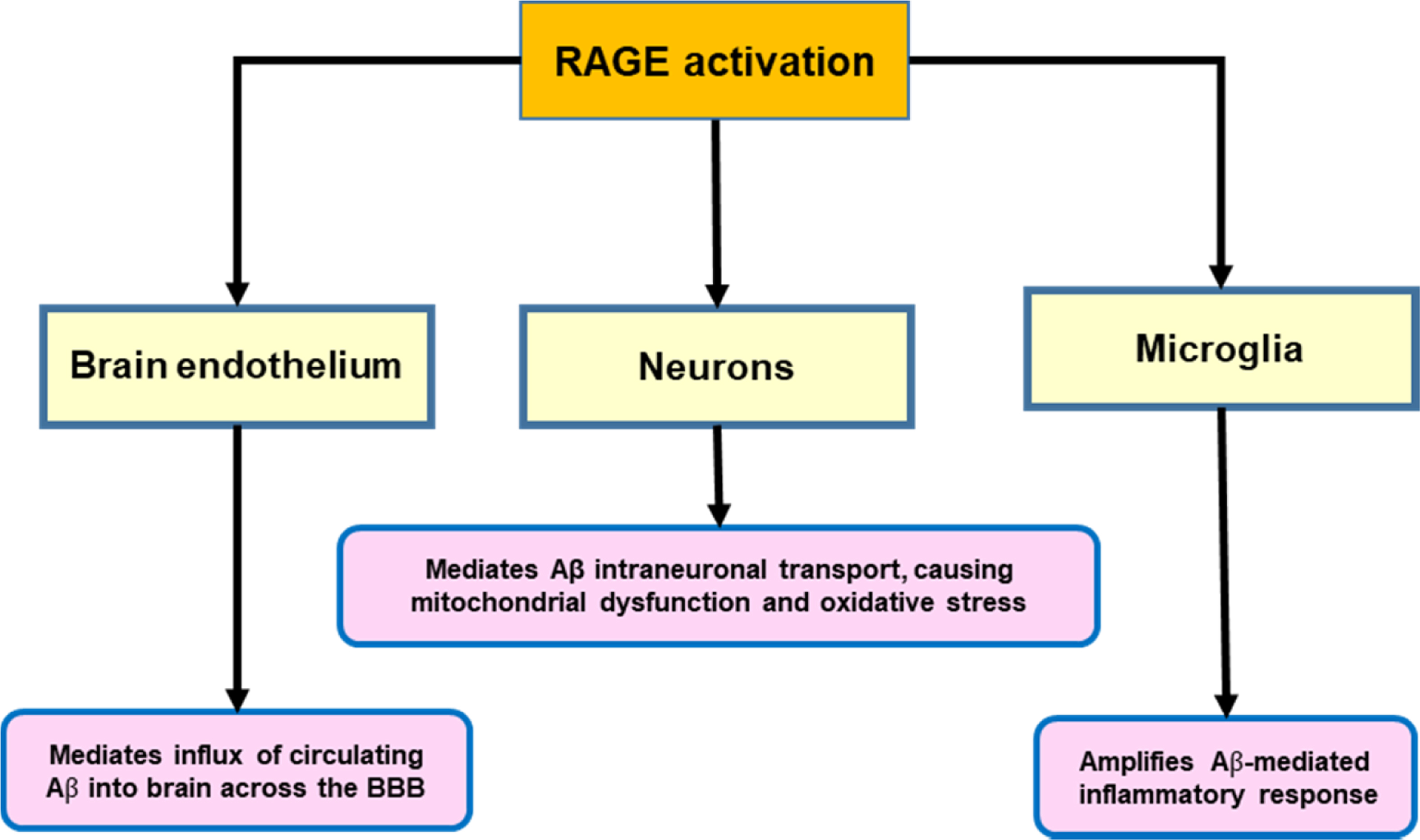
Obviously, in the diabetic population will be enhanced levels of AGEs and other RAGE ligands in comparison with in nondiabetic subjects, and, consequently, activation of this receptor will be significantly increased.
The RAGE specific inhibitor, FPS-ZM1, reduced Aβ in the brain and prevented Aβ-induced oxidative stress in vivo [ ]. Another small-molecule inhibitor of RAGE, azeliragon (TTP488), was tested in mild AD patients (Clinical Trial no: NCT02080364), but the phase III study terminated due to a lack of efficacy. Another phase II study is being recruited to test the drug in patients with mild AD and impaired glucose tolerance (Clinical Trial no: NCT03980730).
3.4
Mitochondrial dysfunction/oxidative stress
Mitochondrial dysfunction/oxidative stress is an essential mechanism accounting for diabetic complications [ ]. Moreover, there is emergent evidence that they play a key role in the development and progression of AD [ ]. Therefore, it is reasonable to postulate that the higher mitochondrial dysfunction and oxidative stress that occurs in diabetes can accelerate the development of AD.
3.5
Angiotensin II
High levels of angiotensin II have been reported to play a role in glucose and insulin regulation and increase the risk of T2D. Also the ACE gene was associated with insulin resistance and inflammatory factors [ ]. The actions of angiotensin II within the CNS are of increasing interest in the context of AD. Angiotensin II may lead to reduction in cerebral blood flow [ ], or enhance the oxidative stress [ ]. Additionally, angiotensin II may play an important role in the metabolism of Aβ [ ] and inhibits the release of acetylcholine from the human temporal cortex [ ].
The level and activity of ACE within the cerebral cortex are generally elevated in AD patients [ ]. In addition, a recent study showed that serum ACE activity could predict logical memory impairment in T2D patients with MCI [ ].
3.6
Hypoglycemia and glycemic variability
Several prospective studies have shown that severe hypoglycemia is a risk factor for cognitive impairment and dementia, in particular in the elderly T2D population [ , ]. Although the signs and symptoms of acute and severe hypoglycemia could be transient and reversed by glucose administration, the data from some clinical and experimental studies showed that hypoglycemia could lead to permanent neuronal damage in regions of the hippocampus [ ]. Furthermore, McNay et al. showed in a murine model that recurrent moderate hypoglycemia can cause synaptic dysfunction even in the absence of neuronal death, particularly in the hippocampal neurons [ ]. However, the effects of recurrent moderate hypoglycemia on cognitive decline and dementia remain to be elucidated in humans.
There is no doubt that cognitive dysfunction affects treatment adherence and diabetes self-management, resulting in poor glycemic control and an increased rate of severe hypoglycemic episodes [ ]. However, the frequency and severity of hypoglycemias do not appear in current questionnaires for the evaluation of the risk of developing dementia, and, therefore, this crucial point should be tackled urgently.
Severe symptomatic hypoglycemic events in T2D patients were not associated with brain atrophy or white matter abnormalities in the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes [ ]. However, it should be noted that the brain MRI variables measured reflect only structure, not function.
Apart from chronic hyperglycemia or hypoglycemic episodes, glycemic variability has to be considered. Rizzo et al. [ ] in a cross-sectional study found a significant association between glycemic variability measured by mean amplitude of glycemic excursions (MAGE) after 48 h of continuous subcutaneous glucose monitoring (CSGM) and cognitive performance impairment. These findings were also observed in a retrospective longitudinal study: a change in MAGE value significantly correlated with Mini-Mental State Examination (MMSE) changes after 2 years of follow-up. This correlation persisted after adjustment for anthropometric and metabolic parameters, such as BMI and HbA1c. In fact, the MAGE value at baseline was an independent predictor of cognitive worsening [ ].
4
Pathways linking dementia, AD, and DR
AD and DR share similar progressive vasodegeneration, glial activation, and neural damage, which arise from impairment of insulin signaling, the presence of low-grade inflammation, and the activation of pathways directly related to chronic hyperglycemia, such as the accumulation of AGEs and oxidative stress [ ]. However, little is known regarding the molecular mediators shared by the brain and the retina in patients with T2D. In this regard, a proteomic study has identified several AD-linked molecular mediators in the diabetic retina [ ] including some significant pathways connecting neurodegenerative and inflammatory processes that are likely to have strong commonality to the degeneration of the brain.
An ongoing project (RECOGNISED; Clinical Trials gov registration no. NCT04281186) funded by the European Commission (H2020 program-GA 847749) is currently investigating the common mechanisms involved in the pathogenesis of DR and cognitive impairment in the T2D population, as well as the usefulness of retinal imaging as a tool for identifying individuals with T2D at a higher risk of developing cognitive decline or dementia.
4.1
Retinal neurodegeneration: Its relationship with cognitive impairment and brain neurodegeneration
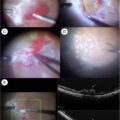
There is robust anatomical, experimental, and functional evidence that neurodegeneration is an early event in the pathogenesis of DR [ ]. There are several methods for measuring retinal neurodegeneration. Multifocal electroretinography (mfERG) and spectral domain optical coherence tomography (SD-OCT) are among the current methods used for this purpose. These noninvasive technologies permit us to detect functional and morphological retinal abnormalities, respectively. However, mfERG is a cumbersome and time-consuming procedure requiring specialized personnel and is mainly reserved for clinical trials. In recent years, microperimetry has emerged as a simple test with even higher sensitivity than mfERG in detecting early functional changes of the retina [ ].
In a nested case-control study (DIALRET project) comprising 35 patients with T2D without cognitive impairment, 35 with MCI, and 35 with AD, members of the RECOGNISED consortium demonstrated that retinal sensitivity assessed by microperimetry significantly correlated with neuroimaging parameters (MRI and PET) and cognitive status [ ]. Overall, the results of this pilot study suggest that retinal sensitivity assessed by microperimetry could be a useful biomarker for identifying patients with T2D who are at risk of developing AD.
In the CNS, the neurovascular unit (NVU) comprises a complex interdependency of vascular cells (endothelium and pericytes), glia, neurons, and professional immune cells. In both the brain and the retina, the NVU constitutes the blood–brain barrier (BBB) and the blood–retinal barrier (BRB), respectively, and maintains homeostasis of the neuropile by restricting the entry of systemic pathogens and neurotoxins as well as by meeting the metabolic demands of neural activity. There is now strong evidence that changes in multiple components of the NVU are a feature of the brain in AD [ ]. However, there is a lack of information regarding the simultaneous impairment of the NVU in brain and retina in T2D patients, and the underlying mechanisms remain to be elucidated.
4.2
Microvascular changes in DR as a risk factor of dementia
Data from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial and in particular from ACCORD-MIND and ACCORD-Eye sub-studies have shown that DR is associated with lower gray matter volume and predicts future cognitive decline in the T2D population [ ]. In addition, it was recently reported that T2D patients with advanced DR (i.e., proliferative DR [PDR] and macular edema [DME]) have a 42% higher risk of incident dementia [ ]. Regarding diabetic nephropathy, there is evidence that microalbuminuria is associated with accelerated cognitive decline in T2D [ , ]. Although this information strongly suggests that T2D patients with microangiopathy (i.e., DR and/or microalbuminuria) present a higher risk of cognitive decline and dementia than T2D patients without microangiopathy, the presence and degree of these complications have never been appropriately considered in the strategies for the identification and stratification of T2D patients at risk of dementia. In fact, causal inferences from these studies on the role of microvascular pathology as reflected by retinal vascular measures in dementia and cognitive decline are limited because most studies have been either cross-sectional, retrospective, or had a short duration of follow-up. On this basis, further evaluation of microvascular changes occurring in DR, and their relationship with those taking place in the brain and the development/progression of cognitive impairment in the T2D population is warranted.
Simó et al. [ ] have proposed an integrative scheme summarizing the two main types of cognitive impairment progression in T2D patients ( Fig. 14.4 ). Insulin resistance, AGEs accumulation, and inflammation are the main pathogenic factors shared by T2D and AD, and, therefore, they are essential in accounting for the increased risk of developing AD in T2D patients. These factors could also influence the increased risk of dementia not related to AD that occurs in T2D. However, micro- and macrovascular disease and the metabolic control, including the rate of severe hypoglycemic events, are perhaps more critical for this slow cognitive decline.
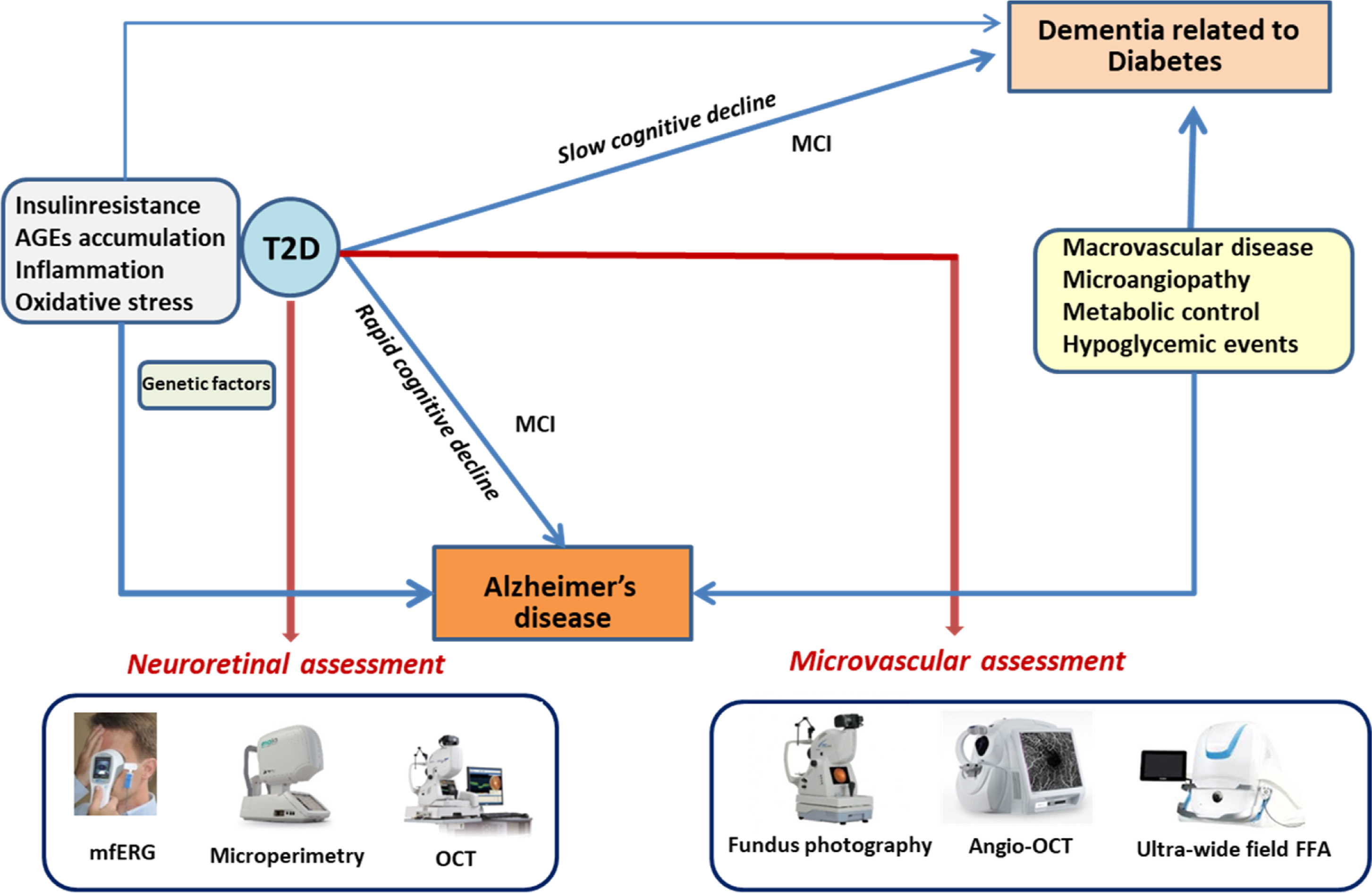
5
Assessment of cognitive decline
In recent years, several neuropsychological questionnaires have been developed for the early detection of cognitive decline.
Basic examination of cognitive evaluation is realized by using the Folstein’s Minimum Mental State Examination (MMSE) [ ]. MMSE is a largely used test to screen for cognitive impairment. A recent work showed that a standard MMSE cut score of 24 (≤23) yielded a sensitivity of 0.58 and a specificity of 0.98 in detecting probable and possible AD across ethnicities. A cut score of 27 (≤26) resulted in an improved balance of sensitivity and specificity (0.79 and 0.90, respectively). In MCI group, the standard cut-off score of 24 yielded a sensitivity of 0.38 and a specificity of 1.00, while raising the cut score to 27 resulted in an improved balance of 0.59 and 0.96 of sensitivity and specificity, respectively [ ].
Most of the authors use MMSE, Hachinski Ischemia Scale [ ], and Clinical Dementia Rating (CDR) [ ] in order to screen for dementia. CDR is more general and establishes five possible stages: 0 = normal; 0.5 = questionable; 1 = mild dementia; 2 = moderate dementia; and 3 = severe dementia. The estimation is based on the performance of the subject in six modalities of cognitive and functional domains: memory, orientation, reasoning, social activities, recreational activities, and personal care.
The AD assessment scales (ADAS-cog) is a relatively short test (30 min) that evaluates cognitive and behavioral dysfunction. It is highly effective to discriminate patients with AD from subjects without cognitive impairment and is considerably more sensitive than MMSE for dementia [ ]. Additionally, ADAS-cog test is used for the evaluation clinical and specific treatment response follow-up.
Recent data showed that a reliable screening for cognitive impairment in T2D can be based on neuropsychological evaluation using Monteral Cognitive Assessment and DSST tests [ ]. Both tests explore short-term memory, visuospatial abilities, attention, concentration, and working memory in T2D patients.
These neuropsychological tests and questionnaires allow the general practitioner to suspect the cognitive impairment. It should be mentioned that these tests are time-consuming taking about an hour to screen for cognitive impairment (10 min for MMSE, 10 min for Montreal Cognitive Assessment, 40 min for ADAS-cog, and 2 min for the DSST test).
Additionally, although these scales have an important role in diagnostic evaluation and follow-up of the patients with dementia, they may be less sensitive for the detection of early or very mild dementias. In these cases, a more exhaustive exploration of the cognitive functions performed by specialized personnel is needed. A complete neuropsychological battery [ ] includes tests sensitive to processing speed, orientation, attention, verbal learning and memory, language, visuoperception, gnosis, praxis, and executive functions, making it time consuming and cumbersome for the daily practice.
Another problem is that the number of patients of whom theoretically the general practitioner or the endocrinologist/diabetologist should evaluate the cognitive function is enormous. In order to facilitate this task, Self-Administered Gerocognitive Examination questionnaires have been proposed as appropriate tools as a first step in a case-finding strategy to detect undiagnosed cognitive impairment [ ].
However, the impairment of QoL and the associated anxiety-depression that occurs in a significant proportion of aging T2D patients has not generally been considered. This is important because apart from influencing the result of neuropsychological tests, the psychological abnormalities related to a decrease in QoL in T2D patients could play a role in aggravating their cognitive decline. In fact, depression was associated with cognitive decline in the ACCORD-MIND study [ ]. Additionally, the prevalence of depression is 2-fold higher in T2D compared with the general population worldwide [ ], and it has recently been reported of 27.5% among T2D patients in the Mediterranean population [ ]. This prevalence rate could even be higher in the older adults with diabetes because depressive symptoms may be overlooked [ ].
5.1
Anthropometric parameters of insulin resistance
There is clear evidence that brain insulin resistance is a crucial pathogenic factor in the development of AD [ ]. In addition, subjects with systemic insulin resistance have an increased risk of AD. A recent study showed that higher HOMA-IR was associated with higher levels of Aβ. APOEε4 carriers had significantly higher levels of Aβ compared to noncarriers. The presence of higher HOMA-IR predicted worse delayed memory performance, suggesting that IR and APOE ε4 are contributing factors to the development of AD pathology in midlife and provide support for targeting insulin function as a potentially modifiable risk factor for AD [ ].
Some genotypes and circulating proteins and phospholipids have been reported useful for identifying subjects at risk of developing AD [ ], but there is no information as to whether they are also useful in the diabetic population. The “omics” high-throughput technologies (genomics, proteomics, and metabolomics), as well as a holistic approach based on systems biology (a methodology based on the computational and mathematical modeling of complex biological systems), could help us in identifying T2D patients at risk of developing AD. However, reliable biomarkers are needed for proposing a cost-efficient model of the cognitive impairment evaluation in T2D patients.
5.2
Brain imaging
T2D patients have brain structural abnormalities (smaller gray and white matter volumes) and alterations in functional connectivity in comparison with their nondiabetic counterparts. In addition, the progression of cerebral atrophy in T2D patients has been linked to accelerated cognitive decline. In this regard, it has been shown that T2D is associated with smaller brain volumes in gray matter.
In addition, the Utrecht Vascular Cognitive Impairment Study Group found that T2D patients show microstructural abnormalities in various white matter pathways. Interestingly, these structural network abnormalities were related to the slowing of information processing speed [ ]. Even more interesting is the fact that before T2D, in the insulin-resistance stage, there is structural brain damage. In this regard, it has been shown that the higher the HOMA the lower the volume of gray matter [ ]. This strongly suggests that the pathways related to insulin signaling rather than blood glucose levels, per se, play a key role in the development of AD in T2D patients.
However, longitudinal studies addressed to examine the prognostic usefulness of all these MRI findings for the prediction of accelerated cognitive decline in T2D patients are needed. It could be of special interest to examine whether brain atrophy is most pronounced in the hippocampus (the earliest and most severely involved brain structure in AD). Indeed, this has been recently investigated by Wisse et al. [ ] who observed greater brain atrophy in T2D patients in comparison with controls, but they were unable to find any specific vulnerability of the hippocampus. In fact, hippocampal insulin resistance is an emerging concept that could play a key role in linking T2D and AD [ ].
In support to this concept, it should be noted that structural brain damage can already be detected in subjects with insulin-resistance without overt [ ]. This strongly suggests that the impairment of insulin signaling rather than blood glucose levels, per se, plays a key role in the development of cognitive decline in T2D patients.
The presence of vascular disease could also participate in brain atrophy. In this regard, Nitkunan et al. showed that brain volume is decreased in the patients with cerebral small vessel disease (CSVD) with respect to normal aging subjects, and this decrease was correlated with cognition decline during 1 year of prospective follow-up [ ]. CSVD includes lacunar infarcts, white matter lesions, and microbleeds. Data so far, suggests that cerebral small vessel disease (CSVD) might be involved in AD [ ]. Nevertheless, future studies are needed in order to reveal the exact role of CSVD in AD.
Another useful tool in AD is the evaluation of brain glucose metabolism by FDG-PET. Cerebral glucose metabolism is largely a measure of synaptic activity and loss of synapses is an early feature of AD that explains the mechanism of progressive cognitive decline [ ]. Patients with AD and MCI show well-documented patterns of reduced 18-fluorodeoxyglucose uptake (FDG-PET) at rest in a network of parietal, posterior cingulate, temporal, and frontal regions [ ].
While there are few existing longitudinal FDG-PET studies in AD and MCI, there is some evidence that FDG-PET accurately predicts subsequent decline and conversion to AD [ ]. However, these studies have not established strong evidence for longitudinal associations between existing cognitive measures and FDG-PET. Landau et al. [ ] found a strong association between FDG-PET and ADAS-cog, indicating that FDG-PET could be useful in clinical trials for selecting subjects who are likely to present a cognitive decline or as an outcome measurement for monitoring clinically relevant change over time. Finally, amyloid positron emission tomography and other molecular imaging technologies could also help us in identifying T2D patients at a high risk of cognitive decline due to AD.
6
New methods for diagnosis and monitoring of cognitive
In recent years, new methods have emerged for the screening and monitoring of the cognitive function in patients with T2D >65 years: the Diabetes Specific Dementia Risk Score (DSDRS) and retinal microperimetry [ , ].
6.1
Diabetes-specific Dementia Risk Score
Diabetes-specific Dementia Risk Score (DSRDS) was proposed in 2013 by Exalto et al. [ ] as a risk score for the prediction of 10 year dementia risk in patients with T2D. This score consists of several clinical and demographic variables (age, gender, education, history of diabetic foot, acute metabolic events, depression, microvascular disease, cardiovascular disease, and cerebrovascular disease) and ranges from a 5% 10 year dementia risk for those with the lowest score up to a 73% risk for the highest score.
The DSDRS was not initially designed as a screening tool. Recently, as part of a European project, MOPEAD study (H2020- IMI-2. GA 115985-2), Ortiz-Zuñiga et al. [ ] evaluated the usefulness of the DSDRS as a screening tool for MCI, alone or combined with MMSE. The cognitive status of the patients was confirmed by neuropsychological tests battery performed by trained neuropsychologists at the memory clinic. The results showed that a cut-off of DSDRS ≥7 had a predictive value of AUC 0.739 (CI 95%, [0.557–0.921]) and of 0.902 (CI 95% [0.840–0.992]) when combined with MMSE [ ], suggesting that DSRDS could be a reliable screening tool for cognitive decline in patients with T2D >65 years. Sensitivity 88.37% CI 95% (78.9–97.9) and specificity 55.26% CI 95% (48.9–60.5).
Additionally, a recent study from another group studied the utility of the DSDRS for lower cognitive performance and found similar results, with a sensitivity of 83.3% and specificity of 54.3% [ ].
6.2
Retinal microperimetry
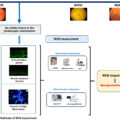
The retina is ontogenically a brain-derived tissue, and it has been suggested that it may provide an easily accessible and noninvasive way of examining the pathology of the brain: “the eye as a window of the brain.” Retinal microperimetry, is a simple, rapid, and noninvasive test that measures retinal sensitivity in terms of the minimum light intensity that patients can perceive when spots of light stimulate specific areas of the retina and also evaluate gaze fixation stability [ , ]. Ciudin et al. showed for the first time that the retinal sensitivity significantly correlated with brain imaging (MRI and PET) and identified patients with MCI and dementia, as confirmed by complete neuropsychological battery.
By adding the gaze fixation parameters to the retinal sensitivity, the probability to identify cognitive impairment significantly increased in an independent manner, suggesting that retinal sensitivity and gaze fixation explore different neuronal circuits and are complementary [ ], converting the retinal microperimetry into a potential useful tool for the screening of MCI in patients with T2D >65 years (with a sensitivity of 72.7% and sensibility of 87.9%).
Furthermore, Ortiz-Zúñiga et al. recently evaluated the usefulness of retinal microperimetry as a monitoring tool for the cognitive function in patients >65 years with T2D [ ]. They found that the worsening in cognitive function went in parallel with worsening of gaze fixation, suggesting this parameter can be a reliable and more subtle tool for the monitoring of the cognitive function in patients with T2D. These results suggest that since retinal sensitivity is a reliable screening tool for diagnosis, the evaluation of gaze fixation could represent a better biomarker for annual follow-up. As previously explained, this finding could be attributed to the fact that the brain areas involved in gaze fixation are not the same as in retinal sensitivity. Retinal sensibility relies on the retina and the neural pathway of vision, which includes the geniculate body and correlated with gray matter volumes in brain MRI in T2D patients with MCI and AD but not white matter [ ], suggesting that could be a marker of neurodegeneration. On the other hand, gaze fixation depends on the complex white matter network [ , ].
Retinal microperimetry, used in the daily clinical practice, as part of the ophthalmological evaluation, is a simple, objective, and rapid test that can be largely used for the monitoring of the cognitive performance, regardless of the psychological status or educational level.
These preliminary data, both on DSDRS and microperimetry, have the potential of changing the current guidelines of clinical practice in patients with T2D if the results are confirmed in larger clinical trials. In this regard, a European project is ongoing (RECOGNISED study, NCT04281186), aimed at validating the usefulness of retinal microperimetry and DSRDS as reliable screening and monitoring tools for cognitive impairment in patients with T2D >65 years. These two tests can be easily implemented and applied at large scale in the daily clinic. If our results are confirmed, the DSDRS could be a screening tool that might easily be implemented and automatically calculated by the electronic medical records of the patients, as part of the daily clinical practice. Additionally, for the annual microperimetry performance in the ophthalmology clinic, an automatic software could provide reliable data on the patients with T2D >65 years that present significant changes in gaze fixation and retinal sensitivity annually, as possible patients at higher risk of progression of their cognitive impairment, on which diabetes care providers should focus and make an accurate diagnosis and management.
7
Conclusions
Cognitive impairment is an emerging condition in T2D but often remains undiagnosed due to lack of simple tools for screening at large scale. This is important because T2D patients with cognitive impairment are more prone to present an impaired self-management of diabetes, poor glycemic control, and an increased incidence of diabetes-related complications. Unfortunately, current guidelines still recommend the use of tests that depend on the mood of the patient and the educational level, with modest sensitivity and specificity for identifying MCI. This represents an important gap because patients with MCI are at risk of progressing to dementia, and the early and reliable identification at large scale will allow the implementation of a patient-centered treatment based on simplifying the regimes and prioritizing antidiabetic treatments with low capacity to provoke hypoglycemia. Recently, new methods have emerged as reliable tools for the cognitive evaluation in patients with T2D > 65 years, such as DSRDS and more importantly, retinal microperimetry, that have the potential to change the current guidelines if preliminary results are confirmed in a large ongoing European trial (RECOGNISED).
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree