Treatment of the Child and Adolescent with Diabetes
Lori Laffel
Cindy Pasquarello
Margaret Lawlor
Diabetes in childhood is a chronic metabolic disorder that results in hyperglycemia. In children, as in adults, energy metabolism is altered as a result either of inadequate insulin secretion or of inadequate insulin action, causing aberrant fuel homeostasis, which affects carbohydrate, protein, and fat metabolism. Diabetes is classified into four types: type 1, type 2, gestational, and other types, including secondary diabetes. The approaches to type 1 and type 2 diabetes in children and adolescents will be reviewed. Gestational diabetes does not generally occur in the pediatric population. Many forms of other types of diabetes, due to causes such as pancreatic hypoplasia or pancreatectomy (secondary to hyperinsulinemia or chronic pancreatitis), behave like and are treated similarly to type 1 diabetes. Another common form of secondary diabetes, cystic fibrosis-related diabetes, appears clinically like either type 1 or type 2 diabetes; readers are referred to recent reviews on this subject (1,2,3,4,5).
DIABETES IN CHILDREN AND ADOLESCENTS: INCIDENCE AND PREVALENCE
Although detailed information on the epidemiology of diabetes appears in Chapter 20, we will summarize fundamental statistics about the occurrence of diabetes in youth, because such information is helpful to practicing clinicians in their work with families and the community. In the United States, the incidence rate of type 1 diabetes is approximately 18 per 100,000 (6,7). The age of peak incidence of type 1 diabetes is gender-specific and coincides with the increased insulin demands of puberty (8). In general, the highest incidence is in the 10- to 14-year-old group and the lowest is in the 0- to 5-year-old group for both genders (8,9). Type 1 diabetes is most likely to develop in girls between the ages of 10 and 12, and boys between the ages of 12 and 14. The prevalence of type 1 diabetes is estimated to be 1.7 cases per 1,000 children and adolescents younger than 20 years old.
While the prevalence is lower with 1 in 2,500 children up to 5 years, it is about 1 in 400 children by 5 to 18 years of age. This translates to approximately 123,000 children and adolescents in the United States who have type 1 diabetes (6,7). In children of all age groups, the overall occurrence of type 1 diabetes has been increasing over the past few decades (9). Estimates of the numbers of youth with type 2 diabetes are less clear. Of the approximately 18,000 children in the United States diagnosed with diabetes each year, 8% to 45% appear to have type 2 diabetes in reports from urban centers in the United States during the last decade (10,11,12,13). The Diabetes SEARCH Study, currently supported by the Centers for Disease Control and Prevention (CDC) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) aims to provide national data on both type 1 and type 2 diabetes in children and adolescents (7; http://www.searchfordiabetes.org).
While the prevalence is lower with 1 in 2,500 children up to 5 years, it is about 1 in 400 children by 5 to 18 years of age. This translates to approximately 123,000 children and adolescents in the United States who have type 1 diabetes (6,7). In children of all age groups, the overall occurrence of type 1 diabetes has been increasing over the past few decades (9). Estimates of the numbers of youth with type 2 diabetes are less clear. Of the approximately 18,000 children in the United States diagnosed with diabetes each year, 8% to 45% appear to have type 2 diabetes in reports from urban centers in the United States during the last decade (10,11,12,13). The Diabetes SEARCH Study, currently supported by the Centers for Disease Control and Prevention (CDC) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) aims to provide national data on both type 1 and type 2 diabetes in children and adolescents (7; http://www.searchfordiabetes.org).
Type 1 Diabetes
Type 1 diabetes is a multifactorial immune-mediated disease characterized by destruction of the pancreatic β-cells by T-cells, leading to a state of insulin deficiency. Type 1 diabetes has long been identified by its previous name, “juvenile diabetes,” implying its common, although not exclusive, occurrence during childhood. Current understanding of its etiology includes an interaction between a genetic predisposition to autoimmunity coupled with an external, environmental trigger leading to autoimmune destruction of the insulin-producing β-cells, resulting in total or near-total deficiency of insulin production (see Chapter 23). Environmental factors, including foods (14,15,16,17), toxins, and viruses, have been suggested as triggers of the autoimmune process in genetically susceptible persons (18,19). Markers of autoimmunity include the presence of circulating autoantibodies to the β-cells, such as cytoplasmic islet cell antibodies (ICAs), insulin autoantibodies (IAAs), glutamic acid decarboxylase (GAD) antibodies, and 64-kilodalton (IA-2) autoantibodies to tyrosine phosphatases (IS-2 and IA-2β) (20,21,22,23,24,25,26,27,28). With improvements in the antibody assays, it has been possible to identify antibodies in the sera of more than 90% of patients with newly diagnosed type 1 diabetes. However, these antibodies are neither necessary nor sufficient for the diagnosis of type 1 diabetes. Identifying the presence of these autoantibodies may be helpful in clinical situations in which it is unclear whether the child has type 1 or type 2 diabetes, particularly with the current epidemic of childhood obesity and type 2 diabetes in youth. Screening for pancreatic autoantibodies in family members of patients with type 1 diabetes also is used as a research tool in studies of the prevention of type 1 diabetes (see Chapter 23). Several human leukocyte antigen (HLA) class II genes have been linked to susceptibility to type 1 diabetes, and some have been linked to a reduced risk of diabetes (29,30,31,32). It is clear that genetic factors, like environmental factors, do not exclusively account for the pathogenesis of type 1 diabetes. In fact, 80% of families of children with newly diagnosed type 1 diabetes do not report any family history of the disease, and the concordance rate in identical twins is only 30% to 50%. The complex interplay of genetics, environment, and autoimmunity as it relates to the etiology, pathogenesis, and possible prevention of type 1 diabetes is the subject of ongoing research.
With the destruction of the majority of β-cells, insulin deficiency results in the onset of the classic symptoms of type 1 diabetes: polyuria, polydipsia, and polyphagia with accompanying weight loss. The stages are outlined below.
Onset: Symptoms such as increased thirst, increased urination, weight loss despite increased appetite.
Honeymoon: Once insulin therapy is initiated, the residual β-cells regain functional capacity, producing some insulin for a short time.
Intensification: Destruction of β-cells continues, and control of blood glucose becomes more difficult.
Total diabetes: All of the insulin-producing β-cells are destroyed, resulting in total insulin deficiency.
Treatment of type 1 diabetes begins at diagnosis and includes insulin therapy, the development of a meal plan, an activity/exercise plan, training in the use of a blood glucose monitor, and family support. Initial self-management training aims to provide the child or adolescent who has diabetes and his or her family with the knowledge, skills, and problem-solving abilities to manage diabetes on a daily basis. This initial education is followed up by ongoing education evolving with duration of diabetes and the growth and development of the child and adolescent (33,34,35).
Type 2 Diabetes
Type 2 diabetes in children and adolescents was identified in the late 1970s and has become a growing medical and public health problem as a result of the burgeoning epidemic of childhood obesity (10,11,12,13,36,37). Like type 2 diabetes in adults, the increase in youth is the direct result of greater caloric intake with decreased caloric expenditure. Youth commonly have poor eating habits, eating high-calorie, high-fat, “super-sized” fast foods; spend an increased amount of time watching television or using computers or playing video games; and are victims of a reduced emphasis on physical education in schools and physical activity in general (38). Almost all youths with type 2 diabetes are overweight and have a positive family history of type 2 diabetes (10,12,37,39). The increasing prevalence of type 2 diabetes has been most marked in ethnic minority groups, including African Americans, Mexican Americans, Asians, Hispanics, and Native Americans (10,12,13) (see Chapter 29).
The first data on type 2 diabetes in youth are from the Pima Indians, the group with the world’s highest prevalence of type 2 diabetes (6). In 1979, the prevalence of type 2 diabetes was 1 in 1,000 children 5 to 14 years of age and 9 in 1,000 youths 15 to 24 years of age (40). By 1996, the prevalence had increased to 22.3 per 1,000 in the 10- to 14-year-old age group and 50.9 per 1,000 in the 15- to 19-year-old group (13). In Northwest Ontario, between 1978 and 1984, the prevalence of type 2 diabetes in Native-American children under the age of 16 years was 2.5 per 1,000, a prevalence higher than that for type 1 diabetes in the white population (11,13,41). In Manitoba, the prevalence of type 2 diabetes among Native-American children studied between 1984 and 1990 was 0.53 per 1,000 children 7 to 14 years of age (11,13,42). In Japanese junior-high-school children, the incidence of type 2 diabetes was recently found to be seven times higher than the incidence of type 1 diabetes (13.9/100,000 vs. 2.07/100,000) and increased more than 30-fold over the past 20 years (43,44).
A study from Cincinnati, Ohio, was the first to document incidence rates of type 2 diabetes in the pediatric population over an extended time (45). One-third of all new cases of diabetes diagnosed between 1982 and 1995 in the 10- to 19-year-old age group were classified as type 2, giving an age-specific incidence of 7.2 per 100,000 per year. In 1992, type 2 diabetes accounted for only 2% to 4% of all newly identified cases of diabetes in patients younger than 19 years of age, but by 1994, 16% of all new cases in children were type 2 diabetes. In the Cincinnati report, 70% of the children with type 2 diabetes were African American, whereas only 10% of the children with type
1 diabetes and 14.5% of the general population were African American (45).
1 diabetes and 14.5% of the general population were African American (45).
In contrast to the similar gender ratio for children with type 1 diabetes, more girls than boys are reported to have type 2 diabetes in studies of type 2 diabetes in children. The ratio of females to males in the Pima Indian population is 2:1; in Ontario Indians, 6:1; in Manitoba Indians, 4:1; and in the predominantly African-American population in Cincinnati, 2:1 (11). Furthermore, diabetes is consistently related to two important variables, obesity and puberty: both states are associated with insulin resistance. Indeed, markers of insulin resistance have recently been identified in 5- to 10-year-old overweight African-American children (46).
Several risk factors are associated with the development of type 2 diabetes: ethnic background, family history of type 2 diabetes, increased blood pressure, increased lipid levels, and obesity (10,12,20,37,45,47,48) (Table 42.1). In addition, acanthosis nigricans, thought to be a cutaneous manifestation of hyperinsulinism, is present in 60% to 70% (vs. the 7% normally seen in this population) of children with type 2 diabetes. These factors are quite similar to the risk factors for type 2 diabetes in adults (10).
TABLE 42.1. Risk Factors Associated with the Development of Type 2 Diabetes in Children and Adolescents | |||
|---|---|---|---|
|
A recently convened task force from the NIDDK, CDC, American Diabetes Association (ADA), and the American Academy of Pediatrics recommend testing for diabetes in youth beginning at 10 years of age or at the onset of puberty if earlier. This recommendation includes testing for type 2 diabetes in children and adolescents if they are overweight (defined as weight >120% of ideal body weight, body mass index [BMI] > 85th percentile for age and gender, or weight for height > 85th percentile), and have any two of the following:
Family history of type 2 diabetes in first- or second-degree relative
Race/ethnicity: African, Hispanic, Asian/South Pacific, and Native-American descent
Signs of insulin resistance such as hypertension, hyperlipidemia, or polycystic ovary syndrome (PCOS) (10).
Determination of the fasting plasma glucose is the preferred means of testing, with a positive diagnosis when fasting plasma glucose is 126 mg/dL or higher. Testing should be repeated every 2 years (20). The initial treatment of type 2 diabetes in youth is dictated by clinical presentation. Diabetic ketoacidosis (DKA) or severe hyperglycemia with nonketotic hyperosmolar hyperglycemic syndrome (NKHHS) requires emergency management per a DKA protocol. Insulin will likely be needed as therapy in these patients even after their recovery from the acute condition. Others who are not ill at diagnosis can initially be treated with medical nutrition therapy and physical activity (see sections below). Unless there is successful weight loss, most patients will require some form of drug therapy. Previously, insulin was the only drug approved by the U.S. Food and Drug Administration (FDA) for use in children and adolescents. Recently, metformin has been approved for use in adolescents 12 years of age and older. Nonetheless, most pediatric endocrinologists use some of the anti-diabetes oral agents to treat children with type 2 diabetes (10,12). The study of metformin in children and adolescents has recently been published, while investigations of other oral hypoglycemic agents in youth are currently under way (10,49). Markers that aid in the differential diagnosis of type 1 and type 2 diabetes in children and adolescents are outlined in Table 42.2.
TABLE 42.2. Differential Diagnosis of Type 1 and Type 2 Diabetes in Children and Adolescents | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
While it is apparent that type 2 diabetes is developing in an increasing number of children, as in adults, the prevalence of this disorder is likely underestimated, given the typical lack of symptoms early in the course of the disease. Thus, the emerging epidemic of type 2 diabetes in children and adolescents presents a challenge not only to the diabetes specialist but also to the primary care provider, who plays a pivotal role in screening for risk factors for development of this disease in the general pediatric population (10,50). There are currently NIH-funded multicenter clinical trials under way to examine treatment and prevention strategies of type 2 diabetes in youth.
Maturity-Onset Diabetes of the Young
An important category of other forms of diabetes is maturity-onset diabetes of the young (MODY). MODY is a monogenic (51,52), autosomal dominant, heterogeneous form of diabetes. It is related to a defect in insulin secretion by the β-cells in the pancreas rather than to an impairment of insulin sensitivity (52,53). It is estimated that about 1% to 3% of people with diabetes have MODY. MODY is characterized by early age of onset (10–30 years of age, although it can be as early as 2 to 3 years of
age), treatment with oral anti-diabetes medications versus treatment with insulin, usually an antibody-negative status, and a diagnosis of diabetes in three or more family generations, often with multiple individuals within those generations. The majority of individuals with MODY are not overweight. This diagnosis should be entertained in children and adolescents with a strong family history of diabetes in multiple generations (54). MODY is reviewed extensively in Chapter 26.
age), treatment with oral anti-diabetes medications versus treatment with insulin, usually an antibody-negative status, and a diagnosis of diabetes in three or more family generations, often with multiple individuals within those generations. The majority of individuals with MODY are not overweight. This diagnosis should be entertained in children and adolescents with a strong family history of diabetes in multiple generations (54). MODY is reviewed extensively in Chapter 26.
THE TEAM APPROACH
In this current era of intensive diabetes control following the release of the Diabetes Control and Complications Trial (DCCT), the team approach to diabetes care remains central to the successful treatment of children and adolescents with diabetes (55,56,57). Care of youth with either type 1 or type 2 diabetes is complex and time-consuming. In this age of managed care and cost-containment, few primary care physicians, including pediatricians, possess the time to care for these patients and keep up with evolving therapies or new technologies.
The expertise required to deliver the numerous components of the diabetes treatment program resides within a multidisciplinary team that works with the child’s family, primary care physician, and school (55,58). The physician-led pediatric diabetes team should be trained in all aspects of pediatric diabetes management and includes a diabetes nurse educator, a dietitian, and a mental health professional—either a social worker or a clinical psychologist. In addition, the team may include an exercise physiologist and subspecialists such as ophthalmologists, podiatrists, nephrologists, gastroenterologists, and others as needed (55). Each team member should appreciate the goals of therapy, the complexities of pediatric diabetes care, the need for individualization, the complications of diabetes, prevention of and early intervention for deteriorating glycemic control, and the impact of the disease on normal childhood and adolescence, as well as on family dynamics.
The process of educating parents and children in diabetes care should begin at the time of diagnosis. Initially, many parents and children or adolescents are overwhelmed and unable to assimilate the extensive body of information of diabetes self-management training. Therefore, it is recommended that self-management training and education be carried out in stages (33,34,35,59,60).
PATIENT AND PARENT EDUCATION
Today, the child with newly diagnosed diabetes is usually treated as an outpatient or during a very brief inpatient admission. Therefore, education goals need to be tailored to the needs of the individual child or adolescent, the family, and the resources of the healthcare team. These initial goals become an important part of the ongoing process of diabetes follow-up care. Central to successful management and attainment of treatment goals are the child or adolescent with diabetes and his or her family, whose needs should receive priority in planning and implementing the treatment program. The child’s primary care provider must be included as a member of the diabetes health-care team. In addition, caregivers such as day-care providers, teachers, school nurses, coaches, grandparents, and babysitters are integral to successful outcomes (Fig. 42.1). This collaborative approach helps the patient receive optimal diabetes care, taking into account his or her age, day-care or school schedule, eating patterns, personality, temperament, family structure, cultural background, and other medical conditions.
Initial goals are limited to imparting an understanding of the fundamental nature of diabetes and how it is treated. Next, diabetes treatment routines need to be integrated into school, sports activities, day care, and family activities. This occurs through practical experience at home and by frequent contact with the diabetes healthcare team. As time passes, most families are ready to learn the intricate details of diabetes self-management necessary for maintaining optimal glycemic control, while coping with the challenges imposed by such things as physical activity, “picky” or selective eating habits, intercurrent illnesses, and other normal variations in a child or adolescent’s daily routine. In addition to imparting facts and teaching practical
skills, diabetes self-management training and education should attempt to promote desirable health beliefs and attitudes in the young person who has to live with a chronic and incurable disease (33,34,35).
skills, diabetes self-management training and education should attempt to promote desirable health beliefs and attitudes in the young person who has to live with a chronic and incurable disease (33,34,35).
For the child, this is often best accomplished in an educational setting such as age-appropriate peer education/support groups or summer camps for children with diabetes. The educational program must match the child’s level of cognitive development and be adapted to the learning style and intellectual ability of the individual child, adolescent, and family (35,61,62,63). We urge that parents be fully involved, and we encourage the diabetes healthcare team to supervise, when appropriate, a gradual and flexible transfer of responsibility from parents to the adolescent, facilitating the normal process of separation and attainment of independence that occurs during the teenage years (64,65,66,67). Ideally, we encourage cooperation around diabetes tasks between the teen and parent with the goal of developing interdependence, because parent involvement has been consistently associated with better medical and behavioral outcomes for youths with diabetes (64,65,66,68).
Continued education is necessary during transitions between developmental stages of childhood, such as at school entry, between the school-age years and adolescence, when the adolescent leaves home for college or for independent living, as well as throughout the lifespan. Some families benefit from reinforcement of teaching skills at home with the help of visiting nurse services, either shortly after diagnosis or during challenging family times. We have established a developmental model of care, education, and psychosocial support through our age-based multidisciplinary clinics.
Beginning with the most vulnerable group of youth and their families, the Pediatric and Adolescent Unit of the Joslin Diabetes Center has implemented a comprehensive, family-focused outpatient program of care for children with type 1 diabetes younger than 8 years of age called the Young Children’s Program (69,70). It is offered once each month, and approximately 400 families with young children have participated to date. Through a systematic assessment of initial needs, we documented that parents of preschool and early school-age children with diabetes are concerned about the following: collisions between diabetes treatment and normal childhood behavior; differentiating symptoms of hypoglycemia from normal behavior and mood swings; and the impact of diabetes on family relationships (69,70). Fear of hypoglycemia surfaced as a primary concern. One of seven of these very young children experienced severe hypoglycemia (70). Thus, families of young children become extraordinarily reliant upon blood glucose monitoring in these young patients who are unable to identify and communicate hypoglycemic symptoms.
We have implemented a similar comprehensive program for school-age children, 8 to 13 years of age, and their families. This group also meets monthly, with the provision of individualized medical care followed by simultaneous separate support groups for parents and for the youth. Similar to the Young Children’s Program, the parent group is facilitated by members of the mental health team, either a child psychologist or social worker, and by members of the medical team, including a physician, a pediatric diabetes specialty nurse, or registered dietitian. Major topics of discussion include transition to middle school, impact of puberty on glycemic control, and review of new technologies along with research updates. An early childhood educator, who coordinates developmentally appropriate activities to insure positive clinic encounters that encourage routine follow-up, supervises the group of youth. Diabetes-specific educational curricula are not routinely discussed.
These comprehensive programs encourage positive interactions between the diabetes healthcare team and the patients along with their families. The positive result of these interactions is evident in our assessment of the Young Children’s Program (70). The program evaluation yielded improved follow-up attendance at diabetes care visits for families who participated in the comprehensive Young Children’s Program compared with infrequent program attendees (70). In addition, the group of patients with improved follow-up care had significantly fewer children with poor control (glycosylated hemoglobin A1c [HbA1c] >9.9%) than the group with infrequent program attendance and follow-up care (P <.05) (70).
Adolescents with diabetes and their families receive multidisciplinary diabetes care as well as medical care and psychosocial support. The program for teens at the Joslin Clinic helps teens and their families devise individualized management programs that fit their lifestyles. Families are helped in the negotiation of acceptable parental involvement in the tasks of diabetes management to sustain adherence to insulin injection routines and blood glucose monitoring (66,71). To provide additional support for the parents as they try to help their adolescents, we initiated a bimonthly evening parent support group facilitated by the multidisciplinary pediatric team. In an initial needs assessment, fear of complications surfaced as a concern in 50% of families, while fear of hypoglycemia was noted by 19%. We stress the importance of continued medical follow-up and frequent blood glucose monitoring for these families to help them overcome these concerns.
We have recently launched a professionally monitored Discussion Board for Teens on the Joslin website. Parents of adolescents also can participate in a Discussion Board specifically for them, as well in the bimonthly discussion group. Parents of adolescents require ongoing help in developing realistic expectations for adolescent blood glucose levels and monitoring behavior, as well as in negotiating an acceptable level of involvement in the diabetes management tasks of their teen. Adolescents with diabetes also require help in developing realistic expectations for blood glucose monitoring and in negotiating with their parents about acceptable levels of involvement in monitoring and insulin routines. The team approach to pediatric diabetes care promotes these goals and remains the accepted standard of care throughout childhood and adolescence (55).
GOALS OF THERAPY
In 1993, the results of the DCCT heralded intensive management or so-called tight control as the standard of care for most patients with type 1 diabetes (56). With scientific evidence showing a marked decrease in the risk of microvascular complications of the eyes, kidneys, and nerves with intensive management, the post-DCCT era emerged (56). Although the study did not enroll patients younger than 13 years of age, there were 195 youth, between the ages of 13 and 17 years old, at entry in the study sample. As a result, diabetes care today utilizes intensive management in the diabetes treatment plans for the majority of children and adolescents with diabetes (72). The theoretical goal of treatment is to restore metabolic function to as near normal as possible while avoiding serious complications of therapy, especially symptomatic hyper- and hypoglycemia. However, the approach to care also incorporates more global goals, such as the normalization of childhood and adolescent development and the maintenance of successful family functioning (34,55,59) (Table 42.3).
TABLE 42.3. Goals of Therapy of the Pediatric and Adolescent Unit of the Joslin Diabetes Center | |
|---|---|
|
Glycemic goals vary for children and adolescents and reflect developmental differences. Since hypoglycemia can have more of an impact on the neurocognitive function of young children (73,74,75,76,77,78,79,80), the ADA position does not support tight glycemic control for children under the age of 2 years and advises caution for children 8 years old and younger (55). Table 42.4 shows the glycemic control goals for youth with diabetes.
TABLE 42.4. Goals for Glycemic Control for Children, Adolescents, and Young Adults with Diabetes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
Several studies indicate that children who develop diabetes during infancy and early childhood may be at increased risk for the subsequent development of cognitive impairment (75,81,82,83,84). Such impairment is presumed to be the result of multiple episodes of severe hypoglycemia, which may be more frequent in very young children because of their hypoglycemic unawareness (85,86,87). Therefore, maintaining very tight control of glucose levels in children with very-early-onset diabetes may be harmful, given the risk of causing recurrent, severe, and potentially debilitating episodes of hypoglycemia that have the potential for neurocognitive sequelae (78,88,89,90). Care must also be taken to avoid poor glycemic control, as one wants to limit the hyperglycemic exposure that is associated with future complications (91). One report raises the question of a correlation between high long-term HbA1c values and intellectual impairment in boys diagnosed below the age of 6 years (92). Furthermore, fear of hypoglycemia among parents who have witnessed severe hypoglycemia in their young children becomes a major deterrent both to achieving optimal control in these children as they mature and in allowing them to experience many normal activities of childhood that require separation from parents (93,94,95). A recent publication reports increased parenting stress, in general and in relation to mealtimes, in families with young children with diabetes, which underscores parental fears of hypoglycemia (96).
The prepubertal child may be relatively protected from microvascular complications, although this is still controversial. Signs of eye or kidney disease are extremely rare in the prepubertal child (97). Diabetic nephropathy, for example, with microalbuminuria used as an index of renal glomerular damage, is significantly less prevalent in children younger than 12 years of age than in those older than 12 years of age matched for duration of diabetes (98). Indeed, it is extremely rare to uncover clinically significant microvascular complications in children younger than the age of 10 years (97,98,99). However, debate continues about the contribution of glycemic control during the prepubertal years to the development of future complications (100). Thus, current clinical care should approach this dilemma by aiming for safe and realistic glycemic targets that limit the occurrence of significant hyperglycemia and hypoglycemia and that match the patient and family’s particular needs.
In children and adolescents, as in adults, individualization of the treatment program is important. In highly motivated families, close monitoring of blood glucose and administering multiple insulin injections or utilizing insulin pump therapy using dosage adjustment algorithms are fairly routine. In other families, simplification of the regimen may be the only key to successful management. Thus, while keeping the general therapeutic principles in mind, the diabetes healthcare team must tailor the goals of treatment to the needs and capabilities of each individual child or adolescent with diabetes and his or her family (33,34,35,59,60).
TYPE 1 DIABETES: GLYCEMIC CONTROL IN THE PEDIATRIC POPULATION AND THE DCCT/EDIC STUDY
Despite multidisciplinary specialty care, glycemic control remains suboptimal in many pediatric diabetes centers worldwide, including our own center. In a recent sample of 300 children with type 1 diabetes, the mean HbA1c was 8.7% ± 1.2%, with 33% achieving an HbA1c level below 8.1% (101). Glycemic control in our population appears quite similar to that reported in other large, cross-sectional studies of pediatric populations (Table 42.5). A multicenter cross-sectional study involving 22 pediatric departments in 18 countries in Europe, Japan, and North America enrolling 2,873 children with type 1 diabetes, reported a mean baseline HbA1c of 8.6 ± 1.7%, with 34% of patients achieving an HbA1c <8.0% (105). Three years later, the mean HbA1c from these centers remained 8.7% ± 1.7% (111). Similarly, a recent cross-sectional nationwide study of 2,579
French children with type 1 diabetes reported an overall mean HbA1c of 8.97% ± 1.98%, with 33% of patients achieving an HbA1c less than 8.0% (105). In the DCCT, 83% of the intensively treated patients achieved HbA1c values of 8% or less, compared with only 20% of the patients receiving standard care. Fewer than 5% of the intensively treated patients achieved HbA1c values <6.05% (56).
French children with type 1 diabetes reported an overall mean HbA1c of 8.97% ± 1.98%, with 33% of patients achieving an HbA1c less than 8.0% (105). In the DCCT, 83% of the intensively treated patients achieved HbA1c values of 8% or less, compared with only 20% of the patients receiving standard care. Fewer than 5% of the intensively treated patients achieved HbA1c values <6.05% (56).
TABLE 42.5. Average Glycemic Control in Children and Adolescents Around the World | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
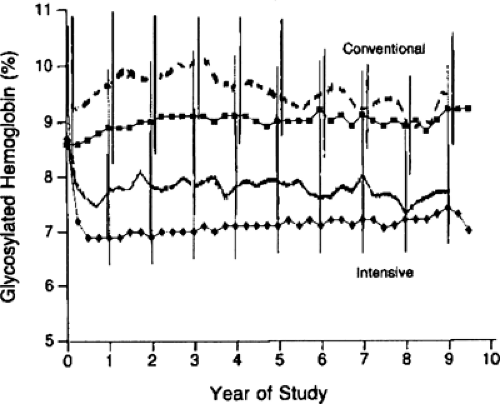
Intensive insulin therapy, compared with conventional therapy, in the DCCT delayed the onset and slowed the progression of long-term complications in both the adolescent and adult cohorts. The subset of the 195 adolescent patients, ages 13 to 17 years at study entry, randomized to intensive therapy experienced a similar reduction in risk for complications to their adult counterparts (112). In contrast to the adolescent cohorts from the DCCT, the adults in both conventionally and intensively treated groups achieved lower HbA1c values both during the DCCT and during the follow-up Epidemiology of Diabetes Interventions and Complications (EDIC) study (56,112,113,114). During the DCCT, intensively treated adults achieved HbA1c values of 7.1% compared with values of 8.9% in conventionally treated adults (Fig. 42.2). Among the 195 adolescents, both intensively and conventionally treated adolescent patients had HbA1c values about 1% higher than those of their older counterparts. At the end of the DCCT, all study patients were encouraged to use intensive insulin therapy. During the EDIC follow-up, both groups of adult patients experienced a change in their glycemic control, with HbA1c values in intensively treated adult patients increasing to 7.9% and HbA1c values in the conventionally treated adults decreasing to 8.1%. Nonetheless, the intensively treated adult patients benefited from their initial exposure to intensive insulin therapy, with a sustained risk reduction in the occurrence of retinopathy and nephropathy during the first 4 years of follow-up in the EDIC study (114). This risk reduction has been confirmed after 7 years of follow-up in EDIC (115).
This significant reduction in risk for microvascular complications was sustained in the intensively treated adolescents when they were followed 4 years after the end of the DCCT (113). One hundred seventy-five of the 195 adolescents initially enrolled in the DCCT participated in the follow-up EDIC Study (115). After the release of the results of the DCCT, the conventionally treated patients were invited to receive intensive insulin therapy. During the first 4 years of follow-up, 50% of conventionally treated adolescents selected multiple daily injections, approximately 17% selected insulin pump therapy, while the remainder continued to receive conventional insulin therapy (113). Among the intensively treated youth during the DCCT, 90% elected to continue intensive insulin therapy during the 4 years of follow-up. Despite the difference in diabetes management between the two groups, glycemic control was identical 4 years after the end of the DCCT, with the group assigned to intensive insulin therapy in the DCCT maintaining a mean HbA1c of 8.4% while those who received conventional insulin therapy achieving a mean HbA1c of 8.5%. Despite this equivalence of glycemic control between the two groups, the previous exposure to intensive insulin therapy was associated with a sustained risk reduction for the occurrence of retinopathy and diabetic nephropathy (113,114).
These differences in glycemic control between adult and adolescent patients during the DCCT and EDIC studies underscore the challenges associated with the management of type 1 diabetes in adolescents. This suggests that adolescents and young adults may have particularly challenging metabolic and behavioral factors that warrant additional study in order to devise successful
treatment programs that can optimize their glycemic control. Issues related to pubertal growth and development, as well as behaviors, likely contribute to these challenges.
treatment programs that can optimize their glycemic control. Issues related to pubertal growth and development, as well as behaviors, likely contribute to these challenges.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree