SUBDURAL EMPYEMA AND SUPPURATIVE INTRACRANIAL PHLEBITIS
BARRY J. HARTMAN AND DAVID C. HELFGOTT
CRANIAL SUBDURAL EMPYEMA
Empyema is an important form of intracranial suppuration, accounting for 15% to 25% of pyogenic intracranial infections (1–3,97). It represents an infectious process that occupies the space between the dura mater and arachnoid surrounding the brain. Left undiagnosed and untreated, subdural empyema is rapidly fatal, so early recognition is critical. In older children and adults, subdural empyema is most often a complication of otorhinologic infection (1,4–19,98). Subdural empyema may also occur as a result of head trauma or surgery, osteomyelitis of the skull, or bacteremic spread from a distant focus of infection. In infants, leptomeningitis is the most common predisposing cause of subdural empyema (11,20,21,97). Males predominate over females, and about 70% of patients are in their second or third decade of life (3–5,7–10,12,13,15,22–26). Patients will present with fever and headache in more than 90% of cases and many will have associated neurologic abnormalities (1,3–9,12,13, 15–19,21,23,24,26–30,99,100,101). The diagnosis of subdural empyema is confirmed using computed tomography (CT) or magnetic resonance imaging (MRI) scanning techniques. However, a strong clinical suspicion despite the lack of evidence for subdural empyema on scans warrants more invasive investigation. Definitive therapy consists of surgical drainage and systemic antibiotics, yet mortality remains as high as 40% in some series (14,21). Spinal subdural empyema also has been described; however, it is quite rare, with fewer than 150 cases reported in the literature (31,102). This condition is addressed briefly later in the chapter. Suppurative intracranial phlebitis is a serious complication of cranial and facial infections and results in thrombosis of the major dural venous sinuses. This is discussed at the end of this chapter.
Historical Perspective
The first comprehensive clinicopathologic descriptions of subdural empyema as a distinct entity were published in the 1940s, although the first definitive report of subdural empyema dates back to 1861 (32,33). This initial report was followed by several more around the turn of the century, with a compilation of 44 cases by Blegvad (32) in 1910. Early names for this disease included “pachymeningitis interna,” “purulent pachymeningitis,” “pia-arachnoid abscess,” “phlegmonous meningitis,” and “subdural abscess,” but these were rejected by Kubik and Adams in favor of “subdural empyema” (8,32). Interestingly, early publications reported a preponderance of subdural empyema secondary to otogenic infections (32). However, because of the compilation of 42 confirmed cases resulting from frontal sinusitis by Courville (33), it has become clear that paranasal sinusitis is the most important causative factor in the development of subdural empyema in older children and adults. During the past 50 years, much has changed in the areas of therapy and diagnosis of subdural empyema. Before the development of antibiotics, subdural empyema was almost always fatal (32,33). Antibiotics, improved diagnosis, and newer surgical techniques have combined to lower the mortality rate to 10% to 40% (1,4,5,7–15,21,22,24,28,29). Physicians depended on their clinical skills and plain roentgenograms of the sinuses and skull to direct their attention to the possibility of intracranial suppuration, until the development of cerebral angiography in the early 1960s, which proved an extremely sensitive method of detecting a subdural collection (13,34). The emergence of CT in the 1970s provided a noninvasive rapid means of visualizing the cranial contents; its reliability, safety, and ease of operation made it the first choice for diagnosis of suspected subdural empyema (2,10,26,35). Recently, MRI has proven even more sensitive than CT (36).
Pathogenesis and Anatomic Considerations
The clinical features of subdural empyema are easily understood if one considers the anatomy of the subdural space with respect to its surrounding structures (Fig. 33.1). The subdural space is normally a potential space rather than an actual space because the dura mater follows the contours of the skull and lies adjacent to the arachnoid and pia mater (37). The ability to form an actual space with fluid collection is greater around the convexities of the cerebral hemispheres where the brain does not approximate the skull as closely as it does around the basal areas (15,22,33).
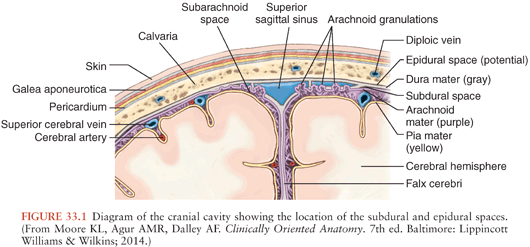
Posteriorly, the tentorium cerebelli is a reflection of dura separating the cerebellum from the cerebral cortex (38). It is an effective barrier to the passage of subdural collection infratentorially, except at its free anterior margin where fluid may seep into the subdural space of the posterior fossa. Only about 10% of subdural empyemas are infratentorial (15,39,40). Medially, the falx cerebri is a reflection of dura extending the length of the cerebrum that separates the cerebral hemispheres (38). Subdural fluid that accumulates between the falx and the arachnoid are known as parasagittal, interhemispheric, or parafalcine subdural empyemas and are usually secondary to surface subdural collections but rarely may be primary (41–43). A subdural empyema may communicate with the contralateral side via the inferior free margin of the falx.
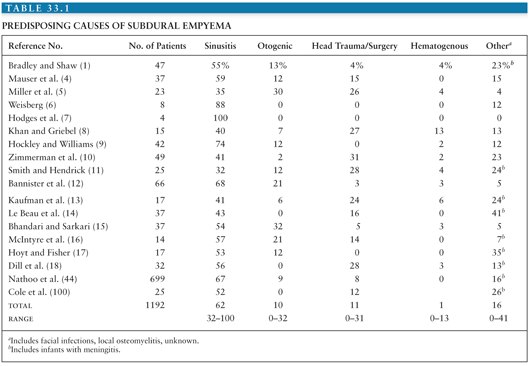
It therefore follows that infections or trauma of the head are the usual causes of subdural empyema. Table 33.1 reviews the conditions predisposing to subdural empyema in series reported during the past two decades (excluding series of only infants). Paranasal sinusitis overwhelmingly predominates as the precipitating factor for the development of subdural empyema (101). The sinusitis almost always involves a frontal sinus, often with other sinuses affected as well. The incidence of subdural empyema following frontal sinusitis is 1% to 2% (45). The frontal and sphenoid sinuses are intimately associated with the dura mater, separated from the dura only by a thin plate of bone.
These sinuses communicate with the maxillary and ethmoid sinuses, which are more anteriorly placed. Because of its position, the frontal sinus is almost always involved in paranasal sinus infection that spreads to the subdural space. In addition, the growing posterior wall of the frontal sinus during puberty has been offered as a possible explanation for the striking age susceptibility for the development of subdural empyema (7,26,46).
Two modes of extension have been proposed for the spread of infection from a frontal sinus to the subdural space: direct and indirect (33). Direct extension involves erosion of the posterior bony wall of the frontal sinus by infection, with further erosion of the underlying dura mater (33). In teenagers primarily, frontal bone osteomyelitis with subperiosteal abscesses (a Pott puffy tumor) can extend directly into the subdural space (47,103). The more likely route is indirect, with extension of infection and associated thrombophlebitis through the mucosal veins of the sinus to the emissary veins that link the facial and dural venous systems (15,33,48). From the dural sinuses, the infection establishes itself in the subdural space at the frontal pole and may spread posteriorly over the convexity, medially into the interhemispheric region, and contralaterally. This extension may create significant pressure on a large area of underlying brain tissue (15,22,49). As stated previously, it is unusual but possible for the empyema to spread infratentorially (15). Further retrograde thrombophlebitis often occurs, involving the valveless, deeper veins of the cerebrum, which in turn may lead to necrosis and infection of brain tissue (10,13,32,33). Subdural empyema secondary to an otogenic source of infection differs only in the site at which pus enters the subdural space. The tympanic cavity is bounded superiorly by the tegmen tympani, a thin plate of bone forming part of the temporal bone of the skull separating the tympanic cavity from the brain (37). Perforating veins pass through this plate of bone to communicate with the superior petrosal venous sinus of the dura (37). In addition, mastoid air cells within the temporal bone surrounding the middle ear communicate with the tympanic cavity and may lie very close to the posterior cranial fossa separated from the dura by slivers of bone (37). Otitis or mastoiditis may, therefore, extend directly into the subdural space via erosion of the tegmen tympani or bone adjacent to the air cells and dura mater or spread infection indirectly by way of a progressive thrombophlebitis of the perforating veins (27,32). Because the venous sinuses into which the veins from the middle ear and mastoid bone drain are within or beneath the tentorium (38), otogenic infection may result in posterior fossa subdural empyema (27). As opposed to subdural empyema secondary to frontal sinusitis, otitis-induced subdural empyema is initially localized posteriorly or on the tentorium (50). Hematogenous spread of bacteria to the subdural space from a distant site of infection is an uncommon cause of subdural empyema, accounting for fewer than 5% of all cases in most series (Table 33.1). Several reports describe the development of subdural empyema via hematogenous spread to a preexisting subdural hematoma (51,52). Subdural empyema is a rare complication following cranial surgery; in one series, subdural empyema occurred in slightly more than 1 in 1,000 craniotomies (53). In a large series of 16,540 craniotomy procedures from 1997 to 2007, only 0.5% had intracranial infection (only 7 cases were pure subdural infections) (104).
In adults, the extension of a subdural empyema from acute purulent meningitis is very unusual. Although there is a subarachnoid inflammatory exudate, the arachnoid is fairly impermeable to the bacterial process occurring adjacent to it (22). Bacterial meningitis in adults is a very unusual cause of subdural empyema, occurring in less than 1% of cases (21,105). However, in infants, meningitis is an important predisposing condition for the development of a subdural empyema (11,20,21,97,100). Subdural empyema occurs in about 2% of infants with bacterial meningitis (54). The pathogenesis is presumably infection of an initially sterile subdural effusion (13,20,21). Such sterile effusions are variably reported as occurring in up to 60% of infants with meningitis (13,20,97).
Clinical Features
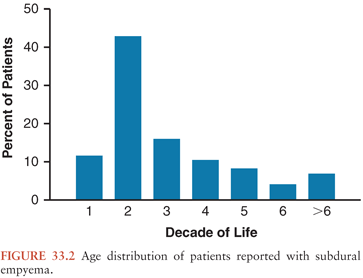
A high clinical suspicion and rapid diagnosis of subdural empyema are critical for a successful outcome. Certainly, an adult with a recent history of sinusitis and a new presentation suggestive of central nervous system (CNS) infection warrants an investigation to exclude subdural empyema. However, in some cases, the antecedent infection is subtle enough to be unrecognized. In others, the concurrent complication of sinusitis with a subdural empyema delays the diagnosis of the latter because symptoms are attributed to the sinusitis. In other cases, the subdural empyema is not suspected because the precipitating cause for the subdural empyema is unknown or arises from a distant focus of infection. Although the clinical presentation may vary, there are key clinical features of subdural empyema that if present should result in its inclusion in one’s initial differential diagnosis. The sex and age distributions of patients with subdural empyema are striking. There is an overrepresentation of men reported in series of patients (mostly adults) with subdural empyema published during the last two decades. In those that report only children, males also predominate. However, in infants, this sex discrepancy may not be so marked (3–5,7–10,12,13,15,16,18,19,22–26,54,99,100,106). Figure 33.2 displays the age distribution of patients reported in series of consecutive patients with subdural empyema. It is clear that most cases occur during the second and third decades of life. As stated earlier, the significant growth of the frontal sinus during puberty has been proposed as an explanation for the uneven age distribution (7,26,46). However, confirmatory analyses comparing patients’ sex, age, and source of infection have not been reported.
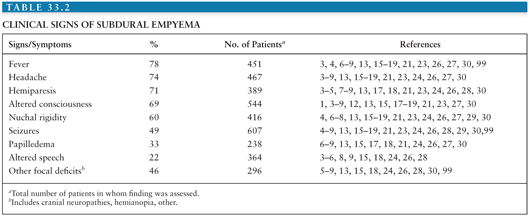
The clinical features of adults with subdural empyema are presented in Table 33.2. Generally, patients have a nonspecific illness for a few days to a few weeks before presentation to the hospital acutely ill (1,5,12,13). However, if the infection is a result of head trauma or surgery, the symptoms may be milder and present more subacutely (10,23,55,56,104). The most common symptoms and signs are headache, fever, neurologic deficit, and stiff neck. Vomiting and malaise are often reported as well (8,15). Seizures, papilledema, and altered level of consciousness ranging from drowsiness and disorientation to coma also occur frequently. These neurologic changes may be presenting signs or, as is often the case, may develop during the course of the illness (6,7,15,28).
Diffuse neurologic signs such as altered level of consciousness, papilledema, and generalized seizures are a result of increased intracranial pressure (ICP) (33,57). Focal neurologic abnormalities such as hemiparesis, jacksonian seizures, dysphasia, and cranial neuropathies may be secondary to local pressure on the underlying cortex by the subdural process (21,27,32,33,41,49,58) and may be precipitated by cortical venous thrombosis with accompanying brain inflammation and infarction (21,22,57). Such focal neurologic signs may help to localize the empyema. This is particularly true in cases of infratentorial subdural empyema that occurs infrequently, but that is easily suspected if cerebellar signs such as ataxia and nystagmus are present (27). Interhemispheric (parasagittal, parafalcine) subdural empyema, usually associated with disease over the convexities but uncommonly occurring alone (43), characteristically produces contralateral leg symptoms, including weakness and focal seizures (41,43,49,58). As the interhemispheric suppuration extends backward over the tentorium and below the occipital lobes, homonymous hemianopia may result (49,58). Subdural empyema overlying one or both convexities yields the most nonspecific neurologic signs. Clues to the involved areas can be (a) contralateral paresis or seizures, (b) aphasia or dysphasia associated with left-sided infection, or (c) cranial neuropathies (32,33,49). The clinical signs of subdural empyema in infants are similar to those in adults. In addition, a bulging anterior fontanelle is a common finding in infants (11,20,54).
Differential Diagnosis
The cardinal features of headache, fever, stiff neck, and neurologic signs are not specific for subdural empyema. The differential diagnosis also includes brain abscess, epidural abscess, meningitis, meningoencephalitis, subdural hematoma, and intracerebral thrombophlebitis (21,48). Of these, the presence of focal neurologic signs makes meningitis much less likely. The presence of nuchal rigidity is unusual in brain abscess and subdural hematoma. Unfortunately, clinical grounds alone do not allow the exclusion of most of these possibilities. Therefore, more specific testing should be undertaken as soon as the diagnosis of subdural empyema is suspected.
Diagnostic Studies
Routine studies such as blood tests and plain roentgenograms are of very little value in patients with suspected subdural empyema. Most patients are found to have a peripheral blood leukocytosis (3,11,13,27,30,33). Plain films of the skull are not useful except to demonstrate a sinusitis or mastoiditis or to show widened sutures in infants (21). In infants, cranial ultrasonography can detect a subdural collection and may differentiate a reactive effusion from a subdural empyema (107). Before the development of CT, cerebral arteriography, with a diagnostic accuracy of 80% to 90%, was the procedure of choice to diagnose subdural empyema (13,15,21,27). Although nearly perfect for the detection of hemispheric and parafalcine subdural collections, the sensitivity of carotid angiograms for posterior fossa subdural empyema was not as great (27). Presently, the safety, ease of application, and reliability of CT and MRI make them the modalities of choice to diagnose subdural empyema.
Computed Tomography and Magnetic Resonance Imaging
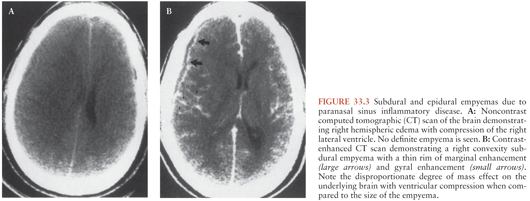
The radiologic evaluation of patients with subdural and epidural empyemas has been revolutionized by the advent of CT in 1972 and MRI in 1984. The introduction of CT has had a major impact on the management and prognosis of subdural and epidural empyemas because CT allows (in a noninvasive manner) earlier and more accurate detection, delineation, and characterization of these extraaxial (extraparenchymal) inflammatory lesions and their associated intraaxial (parenchymal) sequelae when compared to carotid arteriography (10). In addition, CT provides an important adjunct to standard clinical parameters in the assessment of the adequacy of patient response to therapy. The CT, findings during the early stages of development of a subdural empyema may be subtle and easily overlooked (10). Noncontrast CT scans typically demonstrate a crescentic hypodense collection over one or both cerebral convexities and/or around the interhemispheric tissue (Fig. 33.3A). Contrast-enhanced CT increases the conspicuity of the collections that represent active inflammatory disease either in the leptomeninges or in the subjacent cerebral cortex (Fig. 33.3B).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








